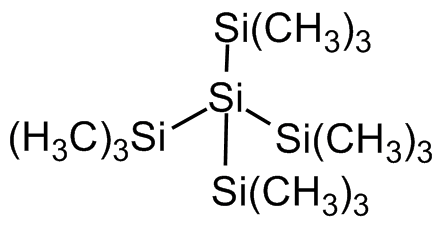Tetrakis(trimethylsilyl)silane
| Code | Size | Price |
|---|
| CDX-T0492-G005 | 5 g | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +4°C, Long term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2,2-Bis(trimethylsilyl)-1,1,1,3,3,3-hexamethyl-trisilane; TTMSS
Appearance:
White to off-white powder.
CAS:
4098-98-0
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H315 - H319 - H335
InChi:
InChI=1S/C12H36Si5/c1-13(2,3)17(14(4,5)6,15(7,8)9)16(10,11)12/h1-12H3
InChiKey:
BOJSDHZZKKYWAS-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 4098-98-0. Formula: (CH3)3Si]4Si. MW: BD9837. Tetrakis(trimethylsilyl)silane (TTMSS) is an organosilicon compound. TTMSS is widely used as a reagent and precursor in organic synthesis and silicon-based chemistry. TTMSS is commonly used as a protecting group for various functional groups in organic synthesis, in deoxygenation reactions to remove oxygen-containing functional groups like ketones, aldehydes, and esters, converting them into the corresponding trimethylsilyl ethers. TTMSS is utilized in silylation reactions to introduce trimethylsilyl groups onto organic compounds, enhancing their volatility and stability for gas chromatography and mass spectrometry analysis. Due to its diverse applications in organic chemistry, Tetrakis(trimethylsilyl)silane has become a valuable tool for synthetic chemists, particularly in the fields of silicon-based chemistry, protecting group strategies, and cross-coupling reactions.
MDL:
MFCD00054859
Molecular Formula:
(CH3)3Si]4Si
Molecular Weight:
320.84
Package Type:
Vial
Precautions:
P302 + P352 - P305 + P351 + P338
Product Description:
Tetrakis(trimethylsilyl)silane (TTMSS) is an organosilicon compound. TTMSS is widely used as a reagent and precursor in organic synthesis and silicon-based chemistry. TTMSS is commonly used as a protecting group for various functional groups in organic synthesis, in deoxygenation reactions to remove oxygen-containing functional groups like ketones, aldehydes, and esters, converting them into the corresponding trimethylsilyl ethers. TTMSS is utilized in silylation reactions to introduce trimethylsilyl groups onto organic compounds, enhancing their volatility and stability for gas chromatography and mass spectrometry analysis. Due to its diverse applications in organic chemistry, Tetrakis(trimethylsilyl)silane has become a valuable tool for synthetic chemists, particularly in the fields of silicon-based chemistry, protecting group strategies, and cross-coupling reactions.
Purity:
>97% (GC)
Signal Word:
Warning
SMILES:
C[Si](C)(C)[Si]([Si](C)(C)C)([Si](C)(C)C)[Si](C)(C)C
Solubility Chemicals:
Soluble in chloroform (100mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) C.H. Ming, et al.; J. Organomet. Chem. 316, 255 (1986) | (2) J. Lalevee, et al.; J. Org. Chem. 72, 6434 (2007) | (3) E. Perret, et al.; J. Phys. Condens. Matter 22, 235102 (2010) | (4) L.D. Funt, et al.; J. Org. Chem. 82, 7583 (2017) | (5) C. Chatgilialoglu, et al.; Chem. Rev. 118, 6516 (2018) | (6) Y. Lee, et al.; J. Nanosci. Nanotechnol. 21, 2139 (2021) | (7) S. George, et al.; Org. Lett. 25, 4345 (2023)