Retatrutide . sodium salt
| Code | Size | Price |
|---|
| AG-CP3-0044-M001 | 1 mg | £120.00 |
Quantity:
| AG-CP3-0044-M005 | 5 mg | £340.00 |
Quantity:
| AG-CP3-0044-M025 | 25 mg | £1,000.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short term: +4°C, Long term: -20°C.
Images
Documents
Further Information
Alternate Names/Synonyms:
LY3437943; GGG Tri-agonist
Appearance:
White lyophilized powder.
CAS:
2381089-83-2 (free acid)
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
Long Description:
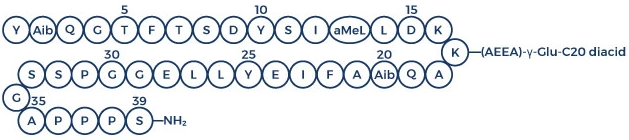
Chemical/Peptide. CAS: 2381089-83-2 (free acid). Formula: C221H342N46O68 . Na. MW: 4731.4 . 23.0. Retatrutide is a novel triple agonist peptide of the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide-1 receptor (GLP-1R). Retatrutide inhibits human GCGR, GIPR and GLP-1R with EC50 values of 5.79, 0.0643 and 0.775 nM, respectively and mouse GCGR, GIPR, and GLP-1R with EC50 values of 2.32, 0.191 and 0.794 nM, respectively. It is an important tool for obesity research. Retatrutide potently activates the GLP-1R signaling pathway to stimulate glucose-dependent insulin secretion through activity at the GIP receptor (GIPR) or the GLP-1R. Retatrutide is a synthetic peptide with glucose-lowering effects. It is an antidiabetic agent against type 2 diabetes (T2D), stimulating insulin and suppressing glucagon secretion in a glucose-dependent manner. Retatrutide was also shown to delay gastric emptying, lower fasting and postprandial glucose concentration, decrease food intake and reduce body weight in patients with type 2 diabetes.
Molecular Formula:
C221H342N46O68 . Na
Molecular Weight:
4731.4 . 23.0
Package Type:
Vial
Product Description:
Retatrutide is a novel triple agonist peptide of the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide-1 receptor (GLP-1R). Retatrutide inhibits human GCGR, GIPR and GLP-1R with EC50 values of 5.79, 0.0643 and 0.775 nM, respectively and mouse GCGR, GIPR, and GLP-1R with EC50 values of 2.32, 0.191 and 0.794 nM, respectively. It is an important tool for obesity research. Retatrutide potently activates the GLP-1R signaling pathway to stimulate glucose-dependent insulin secretion through activity at the GIP receptor (GIPR) or the GLP-1R. Retatrutide is a synthetic peptide with glucose-lowering effects. It is an antidiabetic agent against type 2 diabetes (T2D), stimulating insulin and suppressing glucagon secretion in a glucose-dependent manner. Retatrutide was also shown to delay gastric emptying, lower fasting and postprandial glucose concentration, decrease food intake and reduce body weight in patients with type 2 diabetes.
Purity:
>98% (HPLC)
Solubility Chemicals:
Soluble in water (5mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept: T. Coskun, et al.; Cell Metab. 34, 1234 (2022) | The novel GIP, GLP-1 and glucagon receptor agonist retatrutide delays gastric emptying: S. Urva, et al.; Diabetes Obes. Metab. 25, 2784 (2023) | LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial: S. Urva, et al.; Lancet 400, 1869 (2022) | Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist a step forward in the treatment of diabetes and obesity? S.A. Doggrell; Expert Opin. Investig. Drugs 32, 355 (2023) | Differentiation of human subcutaneous adipocytes and measurement of lipolytic function induced by GIP or LY3437943: A. Regmi & W. Roell; STAR Protoc. 4, 102304 (2023) | Gut hormone co-agonists for the treatment of obesity: from bench to bedside: R. Nogueiras, et al.; Nature Metab. 5, 933 (2023) (Review)