anti-ANGPTL4 (human) pAb
| Code | Size | Price |
|---|
| AG-25A-0038-C100 | 100 ug | £173.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Rabbit
Antibody Clonality: Polyclonal
Regulatory Status: RUO
Target Species: Human
Applications:
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Western Blot (WB)
Shipping:
-20°C
Storage:
-20°C
Images
Further Information
Alternate Names/Synonyms:
Angiopoietin-like Protein 4; FIAF; Fasting-induced Adipose Factor; HFARP; Hepatic Fibrinogen/Angiopoietin-related Protein
Concentration:
1mg/ml
EClass:
32160000
Form (Short):
liquid
Formulation:
Liquid. 0.2µm-filtered solution in PBS, pH 7.4. Contains no preservatives.
Handling Advice:
After opening, prepare aliquots and store at -20°C.Avoid freeze/thaw cycles.
Immunogen:
Recombinant human ANGPTL4.
Long Description:
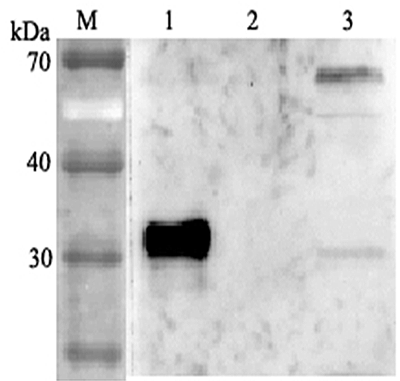
Polyclonal Antibody. Recognizes human ANGPTL4. Detects a band of ~35kDa and 62kDa by Western blot. Source: Rabbit. Applications: ELISA, WB. Liquid. 0.2µm-filtered solution in PBS, pH 7.4. Contains no preservatives. ANGPTL4 mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorgenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7.
NCBI, Uniprot Number:
Q9BY76
Package Type:
Plastic Vial
Product Description:
ANGPTL4 mainly expressed in endothelial cells (hypoxia-induced). Regulates angiogenesis and modulates tumorgenesis and directly regulates lipid, glucose, and energy metabolism. Inhibits proliferation, migration, and tubule formation of endothelial cells and reduces vascular leakage. ANGPTL4 is a protein consisting of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (FLD). Both domains have distinct biological functions. The coiled-coil domain is responsible for the inhibitory effects on lipoprotein lipase (LPL) converting the active form of LPL into an inactive form, and the FLD domain mediates its antiangiogenic functions. The coiled coil and the FLD domains are separated by a short linker that can be cleaved after secretion. ANGPTL4 appears on the cell surface as the full-length form, where it can be released by heparin treatment. ANGPTL4 protein is then proteolytically cleaved by proprotein convertases (PCs), including furin, PC5/6, paired basic amino acid-cleaving enzyme 4, and PC7.
Source / Host:
Rabbit
Specificity:
Recognizes human ANGPTL4. Detects a band of ~35kDa and 62kDa by Western blot.
Transportation:
Non-hazardous
UNSPSC Category:
Primary Antibodies
UNSPSC Number:
12352203
Use & Stability:
Stable for at least 6 months after receipt when stored at -20°C.
References
Angiopoietin-like-2 and -3 act through their coiled-coil domains to enhance survival and replating capacity of human cord blood hematopoietic progenitors: H.E. Broxmeyer, et al.; Blood Cells Mol. Dis. 48, 25 (2012)