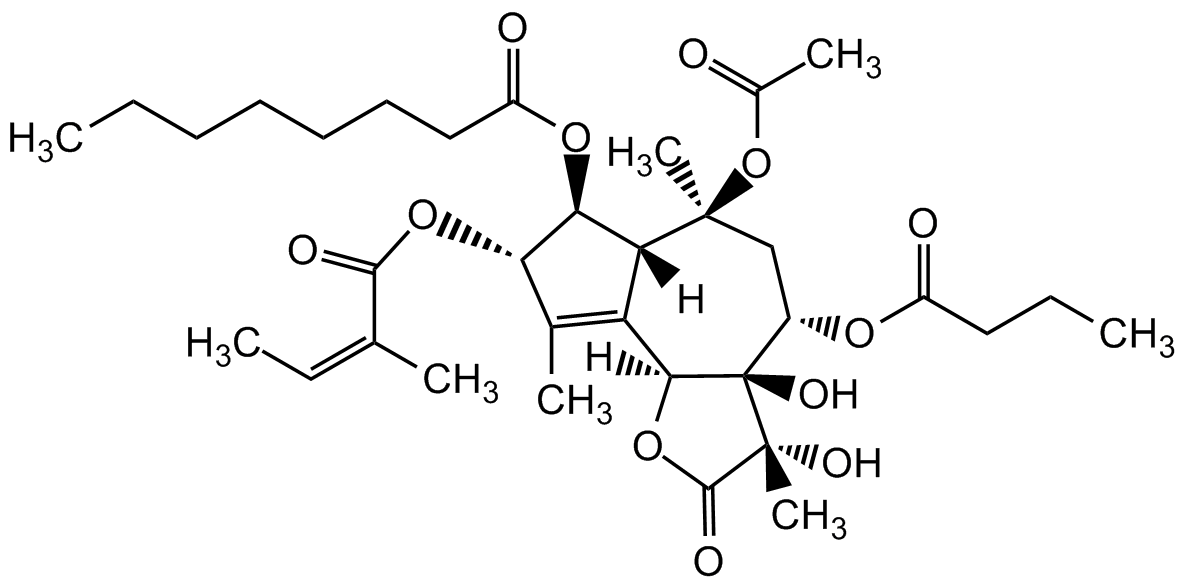
Thapsigargin
Product Code: AG-CN2-0003
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0003-M001 | 1 mg | £70.00 |
Quantity:
| AG-CN2-0003-M005 | 5 mg | £250.00 |
Quantity:
| AG-CN2-0003-M010 | 10 mg | £380.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
Colorless to white amorphous powder.
CAS:
67526-95-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Keep coo
Hazards:
H315, H319, H334, H335
InChi:
InChI=1S/C34H50O12/c1-9-12-13-14-15-17-24(37)43-28-26-25(20(5)27(28)44-30(38)19(4)11-3)29-34(41,33(8,40)31(39)45-29)22(42-23(36)16-10-2)18-32(26,7)46-21(6)35/h11,22,26-29,40-41H,9-10,12-18H2,1-8H3/b19-11-/t22-,26+,27-,28-,29-,32-,33+,34+/m0/s1
InChiKey:
IXFPJGBNCFXKPI-FSIHEZPISA-N
Long Description:
Chemical. CAS: 67526-95-8. Formula: C34H50O12. MW: 650.8. Isolated from Thapsia garganica. Intracellular Ca2+ signaling probe. Specific and sensitive inhibitor of SERCA. Non-TPA/PMA tumor promoter. Histamine release inducer. Apoptosis inducer. Mitochondrial dysfunction inducer. NOS modulator. Angiogenesis inhibitor. Stimulator of arachidonic acid metabolism in macrophages. Autophagy inducer. TRAIL sensitizer.
MDL:
MFCD00083511
Molecular Formula:
C34H50O12
Molecular Weight:
650.8
Other data:
Preparation of 1 mM stock solution: Dissolve 0.65 mg thapsigargin in 1 ml solvent.
Package Type:
Vial
Precautions:
P261, P264, P271, P280, P312
Product Description:
Intracellular Ca2+ signaling probe [1]. Specific and sensitive inhibitor of SERCA [2]. Non-TPA/PMA tumor promoter [3]. Histamine release inducer [4]. Apoptosis inducer [5, 6, 7, 8]. Mitochondrial dysfunction inducer [6, 8]. NOS modulator [9]. Angiogenesis inhibitor [10]. Stimulator of arachidonic acid metabolism in macrophages [11]. Autophagy inducer [12, 13]. TRAIL sensitizer [14]. Inducer of ER-stress in the brain.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
O=C1O[C@@]2([H])C3=C(C)[C@H](OC(/C(C)=CC)=O)[C@@H](OC(CCCCCCC)=O)[C@]3([H])[C@@](C)(OC(C)=O)C[C@H](OC(CCC)=O)[C@]2(O)[C@@]1(O)C
Solubility Chemicals:
Soluble in DMSO or 100% ethanol.
Source / Host:
Isolated from Thapsia garganica.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases: M. Treiman, et al.; Trends Pharmacol. Sci. 19,131 (1998) | Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations: Y. Sagara, & G. Inesi; J. Biol. Chem. 266, 13503 (1991) | Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis: H. Hakii, et al. J. Cancer. Res. Clin. Oncol. 111, 177 (1986) | On the mechanism of histamine release induced by thapsigargin from Thapsia garganica L: S.A. Patkar, et al.; Agents Actions 9, 53 (1979) | Thapsigargin, a Ca(2+)-ATPase inhibitor, depletes the intracellular Ca2+ pool and induces apoptosis in human hepatoma cells: A. Tsukamoto & Y. Kaneko; Cell Biol. Int. 17, 969 (1993) | Thapsigargin induces mitochondrial dysfunction and apoptosis in the mastocytoma P815 cell line and in mouse thymocytes: J.P. Beaver & P. Waring; Cell Death Differ. 3, 415 (1996) | Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells: B. Tombal, et al.; Prostate 43, 303 (2000) | Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis: J.R. Hom, et al.; J. Cell Physiol. 212, 498 (2007) | Thapsigargin, a Ca(2+)-ATPase inhibitor, relaxes rat aorta via nitric oxide formation: H. Moritoki, et al.; Life Sci. 54, PL153 (1994) | Thapsigargin inhibits angiogenesis in the rat isolated aorta: studies on the role of intracellular calcium pools: N. Shukla, et al.; Cardiovasc. Res. 49, 681 (2001) | Stimulation of arachidonic acid metabolism in rat peritoneal macrophages by thapsigargin, a non-(12-O-tetradecanoylphorbol-13-acetate) (TPA)-type tumor promoter: K. Ohuchi, et al.; Cancer Res. Clin. Oncol. 113, 319 (1987) | Regulation of autophagy by the inositol trisphosphate receptor: A. Criollo, et al.; Cell Death Differ. 14, 1029 (2007) | AMPK-independent induction of autophagy by cytosolic Ca2+ increase: A. Grotemeier, et al.; Cell. Signal. 22, 914 (2010) | Novel HTS strategy identifies TRAIL-sensitizing compounds acting specifically through the Caspase-8 apoptotic axis: D. Finlay, et al.;PLoS One 5, e13375 (2010) | Glucose starvation induces cell death in K-ras-transformed cells by interfering with the hexosamine biosynthesis pathway and activating the unfolded protein response: R. Palorini, et al.; Cell Death Dis. 4, e732 (2013) | Development of an Enhanced Phenotypic Screen of Cytotoxic T-Lymphocyte Lytic Granule Exocytosis Suitable for Use with Synthetic Compound and Natural Product Collections: Z. Zhao, et al.; J. Biomol. Screen 21, 556 (2016) | Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane: K.L. O'Neill, et al.; Genes Dev. 30, 973 (2016) | Disulfide bond disrupting agents activate the unfolded protein response in EGFR- and HER2-positive breast tumor cells: R.B. Ferreira, et al.; Oncotarget 8, 28971 (2017) | Unbiased proteomic screening identifies a novel role for the E3 ubiquitin ligase Nedd4-2 in translational suppression during ER stress: D.E. Eagleman, et al.; J. Neurochem. ahead of print (2020)
Related Products
| Product Name | Product Code | Supplier | Phorbol 12-myristate 13-acetate | AG-CN2-0010 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|