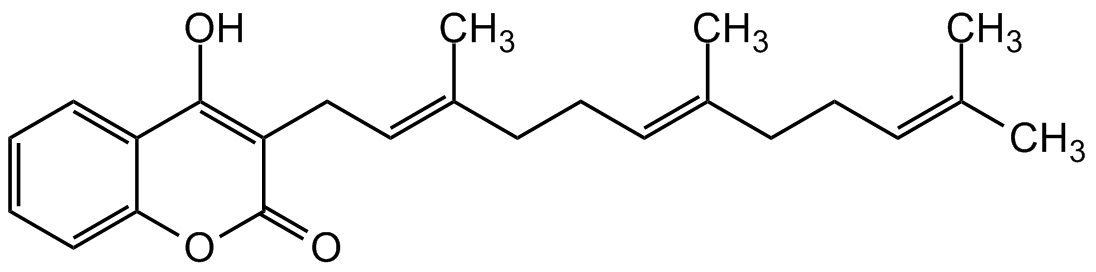
Ferulenol
Product Code: AG-CN2-0011
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0011-M001 | 1 mg | £50.00 |
Quantity:
| AG-CN2-0011-M005 | 5 mg | £150.00 |
Quantity:
| AG-CN2-0011-M010 | 10 mg | £250.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4-Hydroxy-3-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-2H-chromen-2-one
Appearance:
White to off-white solid.
CAS:
6805-34-1
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C24H30O3/c1-17(2)9-7-10-18(3)11-8-12-19(4)15-16-21-23(25)20-13-5-6-14-22(20)27-24(21)26/h5-6,9,11,13-15,25H,7-8,10,12,16H2,1-4H3/b18-11+,19-15+
InChiKey:
NJJDBBUWWOAOLD-CFBAGHHKSA-N
Long Description:
Chemical. CAS: 6805-34-1. Formula: C24H30O3. MW: 366.5. Isolated from Ferula communis. Prenylated 4-hydroxycoumarin. Anti-tumor compound. Cytotoxic. Stimulator of tubulin polymerization in vitro. Inhibitor of colchicine binding to tubulin. Antitubercular antibiotic with potent antibacterial activity. Anti-coagulant, pro-haemorrhagic compound with higher activity than warfarin. Shows hepatocyte toxicity. Disrupts mitochondrial membrane potential.
MDL:
MFCD02183471
Molecular Formula:
C24H30O3
Molecular Weight:
366.5
Package Type:
Vial
Product Description:
Prenylated 4-hydroxycoumarin. Anti-tumor compound [2]. Cytotoxic [2]. Stimulator of tubulin polymerization in vitro [2]. Inhibitor of colchicine binding to tubulin [2]. Antitubercular antibiotic with potent antibacterial activity [3]. Anti-coagulant, pro-haemorrhagic compound with higher activity than warfarin [4]. Shows hepatocyte toxicity [1, 4]. Disrupts mitochondrial membrane potential [5, 6]. Potent L-malate:quinone oxidoreductase (PfMQO) inhibitor in Plasmodium falciparum. Antimalarial.
Purity:
>97% (HPLC)
SMILES:
OC(C1=CC=CC=C1O2)=C(C/C=C(C)/CC/C=C(C)/CC/C=C(C)/C)C2=O
Solubility Chemicals:
Soluble in 100% ethanol, methanol or DMSO.
Source / Host:
Isolated from Ferula communis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L: O. Fraigui, et al.; Vet. Hum. Toxicol. 44, 5 (2002) | Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol: C. Bocca, et al.; Planta Med. 68, 1135 (2002) | Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis): G. Appendino, et al.; J. Nat. Prod. 67, 210 (2004) | Characterization of anti-coagulant properties of prenylated coumarin ferulenol: M. Monti, et al.; Biochim. Biophys. Acta 1770, 1437 (2007) | Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle: M. Lahouel, et al.; BBRC 355, 252 (2007) | Disruption of mitochondrial membrane potential by ferulenol and restoration by propolis extract: antiapoptotic role of propolis: B.H. Nadia, et al.; Acta Biol. Hung. 60, 385 (2009) | Biochemical studies of membrane bound Plasmodium falciparum mitochondrial L-malate:quinone oxidoreductase, a potential drug target: E.D. Hartuti, et al.; BBA Bioenerg. 1859, 191 (2018)
Related Products
| Product Name | Product Code | Supplier | Beauvericin | AG-CN2-0043 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubratoxin A | AG-CN2-0092 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||