PK 11195
| Code | Size | Price |
|---|
| AG-CR1-0008-M010 | 10 mg | £55.00 |
Quantity:
| AG-CR1-0008-M050 | 50 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
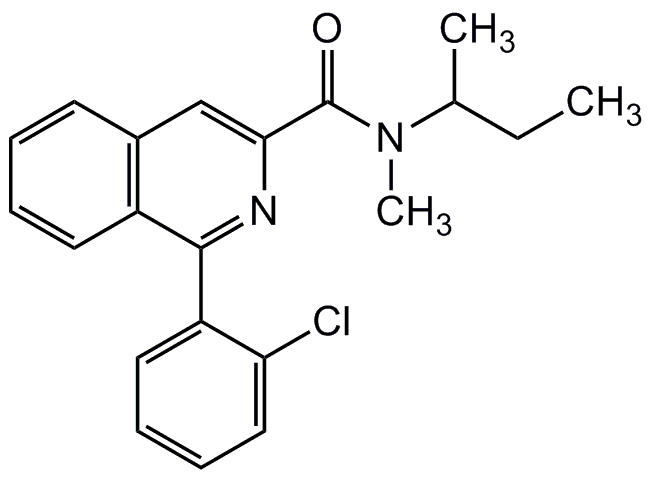
1-(2-Chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide
Appearance:
White to off-white solid.
CAS:
85532-75-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Hazards:
H302, H341
InChi:
InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3
InChiKey:
RAVIZVQZGXBOQO-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 85532-75-8. Formula: C21H21ClN2O. MW: 352.9. Selective peripheral benzodiazepine antagonist. Apoptosis enhancer. Glucose-induced insulin secretion inhibitor. Induces mitochondria cytochrome c release. Anticancer compound. Antiproliferative. Pharmacological tool in autophagy.
MDL:
MFCD00069334
Molecular Formula:
C21H21ClN2O
Molecular Weight:
352.9
Package Type:
Vial
Precautions:
P264, P270, P281, P301, P312
Product Description:
Selective peripheral benzodiazepine antagonist [1, 2]. Apoptosis enhancer [3, 5, 8]. Glucose-induced insulin secretion inhibitor [4]. Induces mitochondria cytochrome c release [6]. Anticancer compound [7]. Antiproliferative [9]. Pharmacological tool in autophagy [10].
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CCC(C)N(C)C(=O)C1=CC2=C(C=CC=C2)C(=N1)C1=CC=CC=C1Cl
Solubility Chemicals:
Soluble in 100% ethanol, methanol, DMSO or dichloromethane; insoluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies: G. Le Fur et al.; Life Sci. 33, 449 (1983) | Dihydropyridine and peripheral type benzodiazepine binding sites: subcellular distribution and molecular size determination: A. Doble et al.; Eur. J. Pharmacol. 119, 153 (1985) | PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection: T. Hirsch et al.; Exp. Cell. Res. 241, 426 (1998) | Inhibition of glucose-induced insulin secretion by a peripheral-type benzodiazepine receptor ligand (PK11195): D. Pujalte, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 362, 46 (2000) | PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepin receptor: R.A. Gonzalez-Polo, et al.; Oncogene 24, 7503 (2005) | Peripheral benzodiazepine receptor ligand, PK11195 induces mitochondria cytochrome c release and dissipation of mitochondria potential via induction of mitochondria permeability transition: J. Li, et al.; Eur. J. Pharmacol. 560, 117 (2007) | The potential anticancer agent PK11195 induces apoptosis irrespective of p53 and ATM status in chronic lymphocytic leukemia cells: A.F. Santidrian et al.; Haematologica 92, 1631 (2007) | Modulation of intracellular Ca2+ signalling in HeLa cells by the apoptotic cell death enhancer PK11195: M. Campanella et al.; Biochem. Pharmacol. 76, 1628 (2008) | Antiproliferative effect of peripheral benzodiazepine receptor antagonist PK11195 in rat mammary tumor cells: S. Mukhopadhyay et al.; Mol. Cell. Biochem. 340, 203 (2010) | Ca2+-dependent autophagy is enhanced by the pharmacological agent PK11195: Pharmacological tools in autophagy: A. Gastaldello, et al.; Autophagy 6 607 (2010)