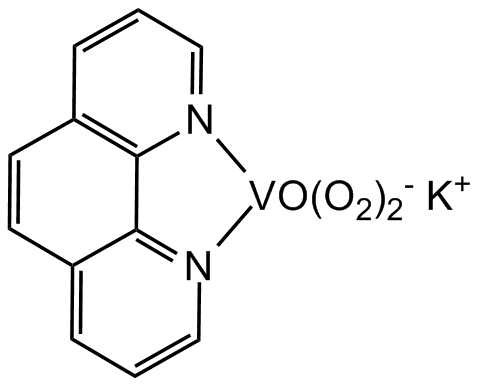bpV(phen)
| Code | Size | Price |
|---|
| AG-CR1-0042-M005 | 5 mg | £55.00 |
Quantity:
| AG-CR1-0042-M025 | 25 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Potassium bisperoxo (1,10-phenanthroline) oxovanadate (V)
Appearance:
Yellow to orange crystalline solid.
CAS:
171202-16-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C12H8N2.K.2O2.O.V/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1;;2*1-2;;/h1-8H;;;;;/q;+1;2*-2;;+3
InChiKey:
PKEQYULPAHCPOP-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 171202-16-7. Formula: K[VO(O2)2C12H8N2] . 3H2O. MW: 350.2 . 54.0. Potent protein phosphotyrosine phosphatase inhibitor. Insulin receptor kinase (IRK) inducer. Insulin mimetic in vitro and in vivo. Apoptosis inducer. ERK inducer. Mitogen-activated protein kinase phosphatase-1 (MKP-1) inducer. Angiogenesis inhibitor. PTEN inhibitor.
Molecular Formula:
K[VO(O2)2C12H8N2] . 3H2O
Molecular Weight:
350.2 . 54.0
Package Type:
Vial
Product Description:
Potent protein phosphotyrosine phosphatase inhibitor [1, 2, 5]. Insulin receptor kinase (IRK) inducer [2]. Insulin mimetic in vitro and in vivo [3, 4]. Apoptosis inducer [6]. ERK inducer [7]. Mitogen-activated protein kinase phosphatase-1 (MKP-1) inducer [7]. Angiogenesis inhibitor [8]. PTEN inhibitor [9].
Purity:
>99% (51V-NMR)
SMILES:
C1[N]2=CC=CC3=C2C2=[N]1C=CC=C2C=C3
Solubility Chemicals:
Soluble in DMSO (1mg/ml) or water (10mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics: B.I. Posner, et al.; J. Biol. Chem. 269, 4596 (1994) | Early signaling events triggered by peroxovanadium [bpV(phen)] are insulin receptor kinase (IRK)-dependent: specificity of inhibition of IRK-associated protein tyrosine phosphatase(s) by bpV(phen): C.J. Band, et al.; Mol. Endocrinol. 11, 1899 (1997) | Hypoglycemic effects of peroxovanadium compounds in Sprague-Dawley and diabetic BB rats: J.F. Yale, et al.; Diabetes 44, 1274 (1995) | In vivo insulin mimetic effects of pV compounds: role for tissue targeting in determining potency: A.P. Bevan, et al.; Am. J. Physiol. 268, E60 (1995) | A role for tyrosine phosphorylation in both activation and inhibition of the insulin receptor tyrosine kinase in vivo: P.G. Drake, et al.; Endocrinology 137, 4960 (1996) | BpV (phen) induces apoptosis of RINm5F cells by modulation of MAPKs and MKP-1: L. Rumora, et al.; BBRC 300, 877 (2003) | Positive regulation of ERK activation and MKP-1 expression by peroxovanadium complex bpV (phen): L. Rumora, et al.; Cell Biol. Toxicol. 20, 293 (2004) | Peroxovanadium compounds as inhibitors of angiogenesis: C. J. Doillon, et al.; Angiogenesis 3, 361 (1999) | Bisperoxovanadium compounds are potent PTEN inhibitors: A.C. Schmid, et al.; FEBS Lett. 566, 35 (2004)