PJ-34
| Code | Size | Price |
|---|
| AG-CR1-0100-M001 | 1 mg | £45.00 |
Quantity:
| AG-CR1-0100-M005 | 5 mg | £75.00 |
Quantity:
| AG-CR1-0100-M025 | 25 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
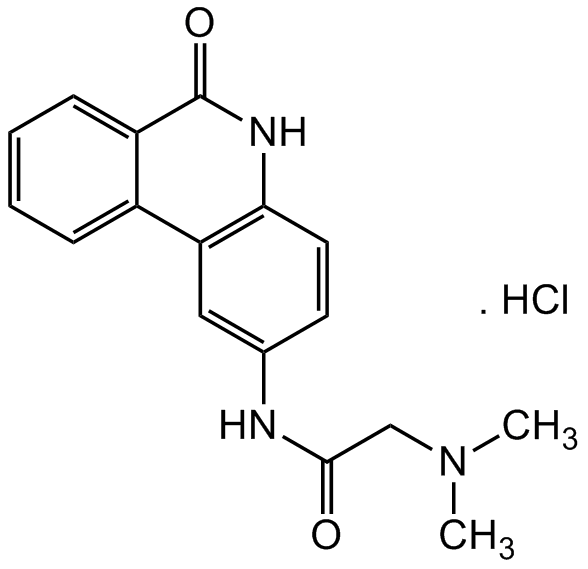
N-(6-Oxo-5,6-dihydro-phenanthridin-2-yl)-N,N-dimethylacetamide . HCl
Appearance:
White to light brown powder.
CAS:
344458-15-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Hygroscopic.
InChi:
InChI=1S/C17H17N3O2.ClH/c1-20(2)10-16(21)18-11-7-8-15-14(9-11)12-5-3-4-6-13(12)17(22)19-15;/h3-9H,10H2,1-2H3,(H,18,21)(H,19,22);1H
InChiKey:
RURAZZMDMNRXMI-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 344458-15-7. Formula: C17H17N3O2 . HCl. MW: 295.2 . 36.5. Potent, water soluble poly(ADP-ribose) polymerase (PARP) inhibitor. Inhibits peroxynitrite-induced cell necrosis. Shows significant, dose-dependent anti-inflammatory effects in a variety of local inflammation models. Provides cardioprotection by decreasing myocardial infarct size. Shows protective effects in models of stroke. Suppresses cell growth in liver cancer cells.
MDL:
MFCD16618379
Molecular Formula:
C17H17N3O2 . HCl
Molecular Weight:
295.2 . 36.5
Package Type:
Vial
Product Description:
Potent, water soluble poly(ADP-ribose) polymerase (PARP) inhibitor. Inhibits peroxynitrite-induced cell necrosis. Shows significant, dose-dependent anti-inflammatory effects in a variety of local inflammation models. Provides cardioprotection by decreasing myocardial infarct size. Shows protective effects in models of stroke. Suppresses cell growth in liver cancer cells.
Purity:
>98% (HPLC)
SMILES:
Cl.CN(C)CC(=O)NC1=CC2=C(NC(=O)C3=CC=CC=C23)C=C1
Solubility Chemicals:
Soluble in DMSO or water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation: F. Garcia Soriano, et al.; Nat. Med. 7, 108 (2001) | Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke: G.E. Abdelkarim, et al.; Int. J. Mol. Med. 7, 255 (2001) | Anti-inflammatory effects of a novel, potent inhibitor of poly (ADP-ribose) polymerase: J.G. Mabley, et al.; Inflamm. Res. 50, 561 (2001) | Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure: P. Pacher, et al.; J. Am. Coll. Cardiol. 40, 1006 (2002) | Novel phenanthridinone inhibitors of poly (adenosine 5'-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents: P. Jagtap, et al.; Crit. Care Med. 30, 1071 (2002) | Myocardial protection by PJ34, a novel potent poly (ADP-ribose) synthetase inhibitor: R. Faro, et al.; Ann. Thorac. Surg. 73, 575 (2002) | PJ34, an inhibitor of PARP-1, suppresses cell growth and enhances the suppressive effects of cisplatin in liver cancer cells: Oncol. Rep. 20, 567 (2008) | The role of PARP activation in glutamate-induced necroptosis in HT-22 cells: X. Xu, et al.; Brain Res. 1343, 206 (2010) | Renal hypoperfusion and impaired endothelium-dependent vasodilation in an animal model of VILI: the role of the peroxynitrite-PARP pathway: R. Vaschetto, et al.; Crit. Care 14, R45 (2010) | Effect of parthanatos on ropivacaine-induced damage in SH-SY5Y cells: T. Zheng, et al.; Clin. Exp. Pharmacol. Physiol. 44, 586 (2017)