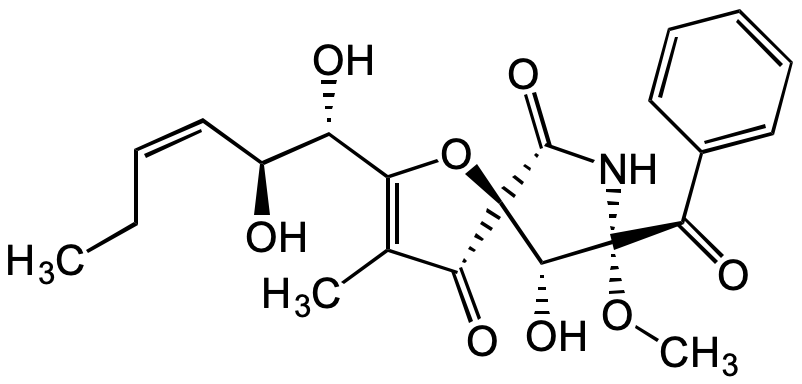
Pseurotin A
Product Code:
BVT-0003
BVT-0003
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0003-M001 | 1 mg | £105.00 |
Quantity:
| BVT-0003-M005 | 5 mg | £320.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
(5S,8S,9R)-8-Benzoyl-2-((1S,2S,Z)-1,2-dihydroxyhex-3-en-1-yl)-9-hydroxy-8-methoxy-3-methyl-1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione
Appearance:
White to off-white powder.
CAS:
58523-30-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319
InChi:
InChI=1S/C22H25NO8/c1-4-5-11-14(24)15(25)16-12(2)17(26)21(31-16)19(28)22(30-3,23-20(21)29)18(27)13-9-7-6-8-10-13/h5-11,14-15,19,24-25,28H,4H2,1-3H3,(H,23,29)/b11-5-/t14-,15-,19+,21+,22+/m0/s1
InChiKey:
SLYDIPAXCVVRNY-UOWMTANKSA-N
Long Description:
Chemical. CAS: 58523-30-1. Formula: C22H25NO8. MW: 431.4. Isolated from Aspergillus fumigatus. Antibiotic. Chitin synthase inhibitor. Cytotoxic. Shows nematicidal activity.
MDL:
MFCD08702705
Molecular Formula:
C22H25NO8
Molecular Weight:
431.4
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Chitin synthase, monoamine oxidase and IgE production inhibitor. Cytotoxic. Shows nematicidal and antileishmanial activity. Antiproliferative against four different glioma cells. Downregulates the expression of tumour glycolytic enzymes pyruvate kinase M2 (PKM2) and lactate dehydrogenase 5 (LDH5) and upregulates the expression of pyruvate dehydrogenase beta (PDHB), adenosine triphosphate synthase beta (ATPB) and cytochrome C, important regulators for tricarboxylic acid cycle and oxidative phosphorylation. Inhibits activation of B-cells and differentiation into the plasma cells
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CC1=C([C@@H](O)[C@@H](O)/C=CCC)O[C@@]2([C@@H](O)[C@](OC)(C(C3=CC=CC=C3)=O)NC2=O)C1=O
Solubility Chemicals:
Soluble in methanol or ethyl acetate; poorly soluble in water.
Source / Host:
Isolated from Aspergillus fumigatus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Novel neuritogenic activities of pseurotin A and penicillic acid: D. Komagata, et al.; J. Antibiot. 49, 958 (1996) | Directed biosynthesis of fluorinated pseurotin A, synerazol and gliotoxin: Y. Igarashi, et al.; J. Antibiot. 57, 748 (2004) | Fumiquinones A and B, nematicidal quinones produced by Aspergillus fumigatus: A. Hayashi, et al.; Biosci. Biotechnol. Biochem. 71, 1697 (2007) | Identification of a hybrid PKS/NRPS required for Pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus: S. Maiya, et al.; ChemBioChem 8, 1736 (2007) | Pseurotin A and its analogues as inhibitors of immunoglobulin E production: M. Ishikawa, et al.; Bioorg. Med. Chem. Lett. 19, 1457 (2009) | Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544: S. Martinez-Luis, et al.; Nat. Prod. Commun. 7, 165 (2012) | Elucidation of Pseurotin Biosynthetic Pathway Points to Trans-Acting C-Methyltransferase: Generation of Chemical Diversity: Y. Tsunematsu, et al.; Angew. Chem. Int. Ed. 53, 8475 (2014) | Angiogenesis Inhibitors and Anti-Inflammatory Agents from Phoma sp. NTOU4195: M.-S. Lee, et al.; J. Nat. Prod. 79, 2983 (2016) | Total syntheses of spirocyclic PKS-NRPS-based fungal metabolites: D. Jo & S. Han; Chem. Comm. 54, 6750 (2018) | Zebrafish-Based Discovery of Antiseizure Compounds from the Red Sea: Pseurotin A2 and Azaspirofuran A: D. Copmans, et al.; ACS Chem. Neurosci. 9, 1652 (2018) | Antiglioma pseurotin A from marine Bacillus sp. FS8D regulating tumour metabolic enzymes: K. Anjum, et al.; Nat. Prod. Res. 32, 1353 (2018) | Treatment of epilepsy using pseurotins and azaspirofurans: D. Copmans, et al.; PCT Int. Appl. WO 2019043019 A1 20190307 (2019) | Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells: O. Vasicek, et al.; Phytomedicine in press (2020)