Antibiotic UK-1
Product Code:
BVT-0013
BVT-0013
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0013-C250 | 250 ug | £95.00 |
Quantity:
| BVT-0013-M001 | 1 mg | £265.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
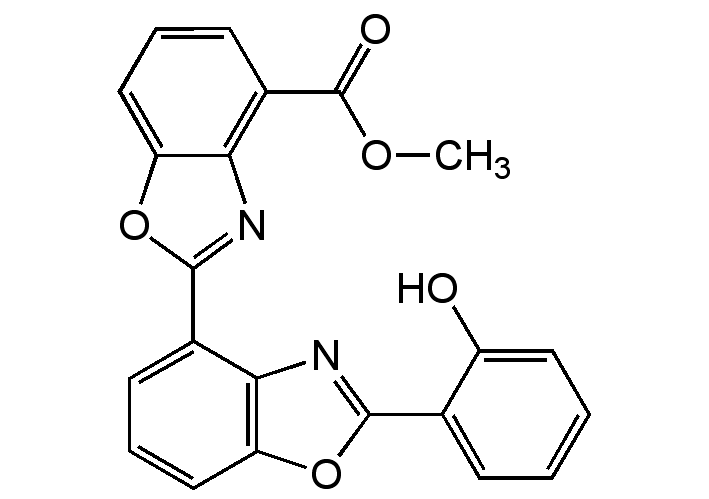
UK1; Methyl 2'-(2-hydroxyphenyl)-[2,4'-bibenzo[d]oxazole]-4-carboxylate; 2'-(2-Hydroxyphenyl)-(2,4'-bibenzoxazole)-4-carboxylic acid methyl ester
Appearance:
White to off-white solid.
CAS:
151271-53-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H319, H332
InChi:
InChI=1S/C22H14N2O5/c1-27-22(26)14-8-5-11-17-19(14)24-21(29-17)13-7-4-10-16-18(13)23-20(28-16)12-6-2-3-9-15(12)25/h2-11,25H,1H3
InChiKey:
NGKIDJMAXLRJRL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 151271-53-3. Formula: C22H14N2O5. MW: 386.4. Isolated from Streptomyces sp. K17/9. Antibiotic. Antifungal. Topoisomerase II (Topo II) inhibitor. Mg2+- and Zn2+-dependent DNA binding agent. Potent anticancer compound.
MDL:
MFCD00921891
Molecular Formula:
C22H14N2O5
Molecular Weight:
386.4
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P304, P340
Product Description:
Antibiotic. Antifungal. Topoisomerase II (Topo II) inhibitor. Mg2+- and Zn2+-dependent DNA binding agent. Potent anticancer compound.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
COC(=O)C1=C2N=C(OC2=CC=C1)C1=CC=CC2=C1N=C(O2)C1=CC=CC=C1O
Solubility Chemicals:
Soluble in DMSO, acetone or dimethylformamide.
Source / Host:
Isolated from Streptomyces sp. K17/9.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
UK-1, a novel cytotoxic metabolite from Streptomyces sp. 517-02. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties: M. Ueki, et al.; J. Antibiot. 46, 1089 (1993) | UK-1, a novel cytotoxic metabolite from Streptomyces sp. 517-02. II. Structural elucidation: K. Shibata, et al.; J. Antibiot. 46, 1095 (1993) | UK-1, a novel cytotoxic metabolite from Streptomyces sp. 517-02. III. Antibacterial action of demethyl UK-1: M. Ueki and M. Taniguchi; J. Antibiot. 50, 788 (1997) | UK-1, a novel cytotoxic metabolite from Streptomyces sp. 517-02. IV. Antifungal action of methyl UK-1: M. Ueki, et al.; J. Antibiot. 51, 883 (1998) | The novel bis(benzoxazole) cytotoxic natural product UK-1 is a magnesium ion-dependent DNA binding agent and inhibitor of human topoisomerase II: M.B. Reynolds, et al.; Bioorg. Chem. 27, 326 (1999) | Evaluation of complexation of metal-mediated DNA-binding drugs to oligonucleotides via electrospray ionization mass spectrometry: M.L. Reyzer, et al.; Nucl. Acids Res. 29, E103 (2001) | Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1: D. Kumar, et al.; Bioorg. Med. Chem. 10, 3997 (2002) | Critical structural motif for the catalytic inhibition of human topoisomerase II by UK-1 and analogs: B.B. Wang, et al.; Bioorg. Med. Chem. Lett. 14, 3221 (2004) | Synthesis and antimicrobial activity of some 5-[2-(morpholin-4-yl)acetamido] and/or 5-[2-(4-substituted piperazin-1-yl)acetamido]-2-(p-substituted phenyl)benzoxazoles: O. Temiz-Arpaci, et al.; Arch. Pharm. 338, 105 (2005) | Synthesis and anticancer evaluation of bis(benzimidazoles), bis(benzoxazoles), and benzothiazoles: S.T. Huang, et al.; Bioorg. Med. Chem. 14, 6106 (2006)
Related Products
| Product Name | Product Code | Supplier | Kendomycin | BVT-0001 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Echinosporin | BVT-0006 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||