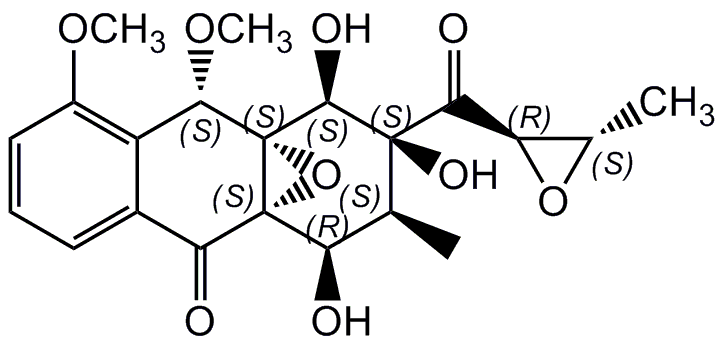
Mensacarcin
Product Code:
BVT-0028
BVT-0028
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0028-M001 | 1 mg | £150.00 |
Quantity:
| BVT-0028-M005 | 5 mg | £425.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Appearance:
White to off-white solid.
CAS:
808750-39-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Hazards:
H302, H312, H319, H332, H351
InChi:
InChI=1S/C21H24O9/c1-8-14(22)20-15(23)10-6-5-7-11(27-3)12(10)17(28-4)21(20,30-20)18(25)19(8,26)16(24)13-9(2)29-13/h5-9,13-14,17-18,22,25-26H,1-4H3/t8-,9-,13+,14+,17?,18-,19+,20+,21+/m0/s1
InChiKey:
WWNXYRCJJRRWAQ-UJLZTCEPSA-N
Long Description:
Chemical. CAS: 808750-39-2. Formula: C21H24O9. MW: 420.4. Isolated from Streptomyces bottropensis. Antibiotic. Antitumor compound. Cytotoxic.
MDL:
MFCD28898968
Molecular Formula:
C21H24O9
Molecular Weight:
420.4
Package Type:
Plastic Vial
Precautions:
P201, P261, P270, P281, P301, P312, P302, P352, P405
Product Description:
Antibiotic. Cytotoxic. Antitumor compound. Anti-melanoma drug lead compound. Effective in BRAF V600E mutation cell lines. Potently induces apoptosis in melanoma cells. Localizes to mitochondria, affects energy metabolism in mitochondria rapidly and activates caspase-3/7-dependent apoptotic pathways. Inhibits cell growth in a borad range of human cancer cell lines. Shows cell type specificity inducing strong cytostasis most effective in cancer types with low oxygen respiration ratios like melanomas.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CO[C@H]1C2=C(OC)C=CC=C2C(=O)[C@@]23O[C@@]12[C@@H](O)[C@@](O)([C@@H](C)[C@H]3O)C(=O)[C@@H]1O[C@H]1C
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Isolated from Streptomyces bottropensis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Towards a total synthesis of the new anticancer agent mensacarcin: synthesis of the carbocyclic core: L.F. Tietze, et al.; Chemistry 10, 5233 (2004) | Cloning and heterologous expression of three type II pks gene clusters from Streptomyces bottropensis: X. Yan, et al.; ChemBioChem 13, 224 (2012) | Insights into the Bioactivity of Mensacarcin and Epoxide Formation by MsnO8: S. Maier, et al.; ChemBioChem 15, 749 (2014) | Functional Characterization of Different ORFs Including Luciferase-Like Monooxygenase Genes from the Mensacarcin Gene Cluster: S. Maier, et al.; ChemBioChem 16, 1175 (2015) | The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells: B. Plitzko, et al.; J. Biol. Chem. 292, 21102 (2017)