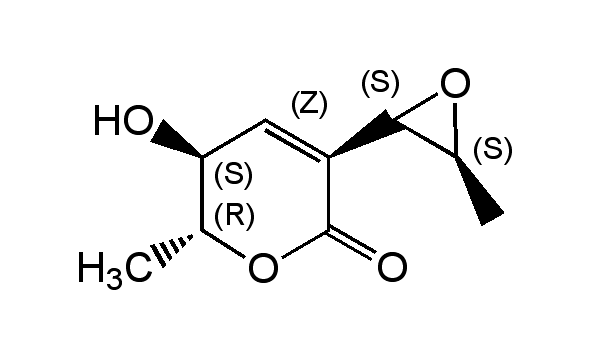
Aspyrone
Product Code:
BVT-0029
BVT-0029
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0029-M001 | 1 mg | £95.00 |
Quantity:
| BVT-0029-M005 | 5 mg | £300.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
(5S,6R)-5-Hydroxy-6-methyl-3-((2S,3S)-3-methyl-oxiran-2-yl)-5,6-dihydro-2H-pyran-2-one
Appearance:
White to off-white solid.
CAS:
17398-00-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from moisture.
Hazards:
H302, H319
InChi:
InChI=1S/C9H12O4/c1-4-7(10)3-6(9(11)13-4)8-5(2)12-8/h3-5,7-8,10H,1-2H3/t4-,5+,7+,8-/m1/s1
InChiKey:
RCAULRNMJFUWRP-HETMPLHPSA-N
Long Description:
Chemical. CAS: 17398-00-4. Formula: C9H12O4. MW: 184.2. Isolated from Aspergillus ochraceus. Antibiotic. Shows nematicidal, insecticidal, antibacterial and antifungal activity. Related to asperlactone and aspinonene.
MDL:
MFCD14635403
Molecular Formula:
C9H12O4
Molecular Weight:
184.2
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P305, P351, P338
Product Description:
Antibiotic. Shows nematicidal, insecticidal, antibacterial and antifungal activity. Related to asperlactone and aspinonene.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
C[C@@H]1OC1C1=C[C@H](O)[C@@H](C)OC1=O
Solubility Chemicals:
Soluble in DMSO, acetone or methanol.
Source / Host:
Isolated from Aspergillus ochraceus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
A new metabolite of Aspergillus melleus: S.D. Mills & W.B. Turner; J. Chem. Soc. (C) 2242 (1967) | Production of 3-(1,2-epoxypropyl)-5,6-dihydro-5-hydroxy-6-methylpyran-2-one by Aspergillus ochraceus Wilhelm: J.H. Moore, et al.; J. Agr. Food Chem. 22, 697 (1974) | J.S.E. Holker, et al.; J.C.S. Perkin Trans. 1, 1397 (1981) | Biosynthesis of aspyrone, a metabolite of Aspergillus melleus: advanced precursor studies to identify the product of the polyketide synthase: J. Staunton & A.C. Sutkowski; J.C.S. Chem. Commun. 1108 (1991) | The polyketide synthase (PKS) of aspyrone biosynthesis: evidence for the enzyme bound intermediates from incorporation studies with N-acetylcysteamine thioesters in intact cells of Aspergillus melleus: J. Staunton & A.C. Sutkowski; J.C.S. Chem. Commun. 1110 (1991) | Biosynthesis of aspinonene, a branched pentaketide by Aspergillis ochraceus, related to aspyrone: J. Fuchser, et al.; J. Chem. Soc. Perkin Trans 1, 1663 (1995) | Effect of fungal metabolites and some derivatives against Tribolium castaneum (Herbst) and Nezara viridula (L.): M. Balcells, et al.; Pestic. Sci. 45, 319 (1995) | Synthesis of chiral aspyrone, a multi-functional dihydropyranone antibiotic: T. Sugiyama, et al.; Biosci. Biotech. Biochem. 59, 1921 (1995) | Aspyrone, a nematicidal compound isolated from the fungus, Aspergillus melleus: Y. Kimura, et al.; Biosci. Biotech. Biochem. 60, 1375 (1996) | Bactericidal and fungicidal activity of Aspergillus ochraceus metabolites and some derivatives: M. Torres, et al.; Pestic. Sci. 53, 9 (1998) | Aspinolide and aspinonene/aspyrone co-metabolites, new pentaketides produced by Aspergillus ochraceus: J. Fuchser & A. Zeeck; Liebigs Ann./Recueil|87 (1997)
Related Products
| Product Name | Product Code | Supplier | Mensacarcin | BVT-0028 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decoyinine | BVT-0030 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CJ-21058 | BVT-0064 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||