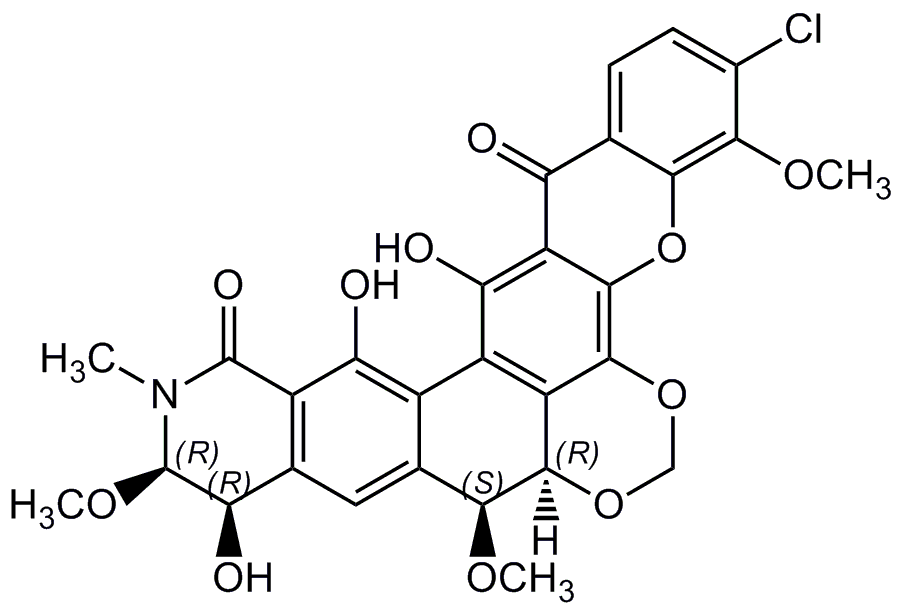
Lysolipin I
| Code | Size | Price |
|---|
| BVT-0037-C500 | 500 ug | £115.00 |
Quantity:
| BVT-0037-M001 | 1 mg | £190.00 |
Quantity:
| BVT-0037-M005 | 5 mg | £555.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Appearance:
Yellow needles.
CAS:
59113-57-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light.
Hazards:
H302, H312, H319, H351
InChi:
InChI=1S/C29H24ClNO11/c1-31-28(36)14-10(19(33)29(31)39-4)7-11-13(20(14)34)15-16-25(22(11)37-2)40-8-41-26(16)27-17(21(15)35)18(32)9-5-6-12(30)24(38-3)23(9)42-27/h5-7,19,22,25,29,33-35H,8H2,1-4H3/t19-,22?,25-,29?/m1/s1
InChiKey:
NEOMIZJYHXSRLV-GLWRQVQYSA-N
Long Description:
Chemical. CAS: 59113-57-4. Formula: C29H24ClNO11. MW: 597.9. Isolated from Streptomyces violaceoniger. Antibiotic. Glycopeptide synthesis inhibitor. Antibacterial, antifungal and anticoccidial. Cytotoxic.
MDL:
MFCD28898532
Molecular Formula:
C29H24ClNO11
Molecular Weight:
597.9
Package Type:
Plastic Vial
Precautions:
P201, P270, P281, P301, P312, P302, P352, P405
Product Description:
Antibiotic. Glycopeptide synthesis inhibitor. Antibacterial, antifungal and anticoccidial. Cytotoxic.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]12OCOC3=C4OC5=C(OC)C(Cl)=CC=C5C(=O)C4=C(O)C(C4=C(O)C5=C(C=C4[C@@H]1OC)[C@@H](O)[C@@H](OC)N(C)C5=O)=C23
Solubility Chemicals:
Soluble in methylene chloride, DMSO or chloroform.
Source / Host:
Isolated from Streptomyces violaceoniger.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Lysolipin I, ein neuer Hemmstoff der bakteriellen Zellwandsynthese.: H. Drautz, et al.; Path. Microbiol. 42, 236 (1975) | Metabolic products of microorganisms, 149. Lysolipin I, a new antibiotic from streptomyces violaceoniger: H. Drautz, et al.; Arch. Microbiol. 106, 175 (1975) | Structure of cervinomycin, a novel xantone antibiotic active against anaerobe and mycoplasma.: A. Nakagawa, et al.; J Antibiot 40, 301 (1987) | Biosynthetic studies on the xanthone antibiotics lysolipins X and I: H. Bockholt, et al.; J. Org. Chem. 59, 2064 (1994) | Experiments on the total synthesis of Lysolipin I. Part II. Michael addition of 1,3-cyclohexanedione to quinone acetals: R.O. Duthaler & U.H.U. Wegmann; Helv. Chim. Acta 67, 1755 (2004) | Isolation of the lysolipin gene cluster of streptomyces Tandae Tu 4042: P. Lopez, et al.; Gene 461, 5 (2010)
Related Products
| Product Name | Product Code | Supplier | Mensacarcin | BVT-0028 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspyrone | BVT-0029 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decoyinine | BVT-0030 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CJ-21058 | BVT-0064 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||