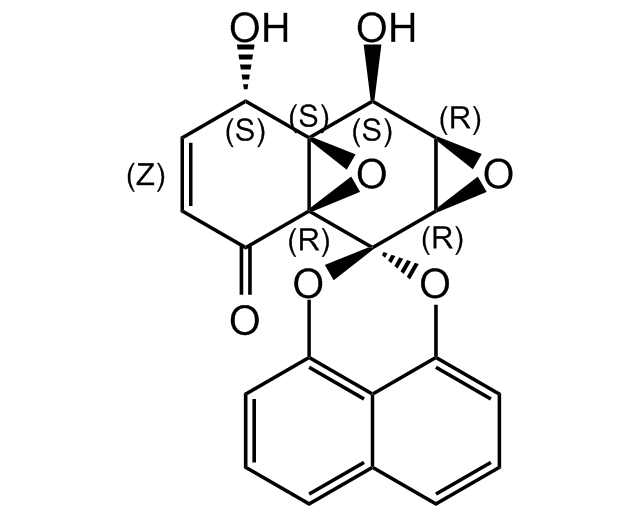
Cladospirone bisepoxide
| Code | Size | Price |
|---|
| BVT-0065-M001 | 1 mg | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Palmarumycin C13; Diepoxin zeta; Antibiotic Sch53514
Appearance:
White to off-white solid.
CAS:
152607-03-9 and 155866-40-3
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H312, H319, H332
InChi:
InChI=1S/C20H14O7/c21-12-7-8-13(22)19-18(12,27-19)16(23)15-17(24-15)20(19)25-10-5-1-3-9-4-2-6-11(26-20)14(9)10/h1-8,12,15-17,21,23H/t12-,15+,16-,17+,18-,19-/m0/s1
InChiKey:
AUWGMDYISSBOED-CCNMWVGKSA-N
Long Description:
Chemical. CAS: 152607-03-9 and 155866-40-3. Formula: C20H14O7. MW: 366.3. Isolated from Sphaeropsidales sp. Antibiotic. Antibacterial and antifungal. Antitumor compound. Germination inhibitor.
MDL:
MFCD09750436
Molecular Formula:
C20H14O7
Molecular Weight:
366.3
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Antibacterial and antifungal. Antitumor compound. Germination inhibitor.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
O[C@H]1C=CC(=O)[C@]23O[C@]12[C@@H](O)[C@H]1O[C@H]1C31OC2=CC=CC3=CC=CC(O1)=C23
Solubility Chemicals:
Soluble in DMSO or methanol:dimethylformamide (1:1).
Source / Host:
Isolated from Sphaeropsidales sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Diepoxins, Novel Fungal Metabolites with Antibiotic Activity: G. Schlingmann, et al.; Tetrahedr. Lett. 34, 7225 (1993) | Production of cladospirone bisepoxide, a new fungal metabolite: F. Petersen, et al.; J. Antibiot. 47, 1098 (1994) | A novel class of antitumor metabolites from the fungus Naitrassia mangiferae: M. Chu, et al.; Tetrahedr. Lett. 35, 1343 (1994) | Biosynthesis of cladospirone bisepoxide, a member of the spirobisnaphthalene family: H.B. Bode, et al.; J. Antibiot. 53, 153 (2000) | UV mutagenesis and enzyme inhibitors as tools to elucidate the late biosynthesis of the spirobisnaphthalenes: H.B. Bode & A. Zeeck; Phytochemistry 55, 311 (2000) | Big effects from small changes: possible ways to explore nature's chemical diversity: H.B. Bode, et al.; Chembiochem 3, 619 (2002) | Natural products derived from naphthalenoid precursors by oxidative dimerization: K. Krohn; Prog. Chem. Org. Nat. Prod. 85, 1-49 (2003) (Review) | Spirobisnaphthalenes from the endophytic fungus Dzf12 of Dioscorea zingiberensis and their antimicrobial activities: X. Cai, et al.; Nat. Prod. Commun. 4, 1469 (2009)