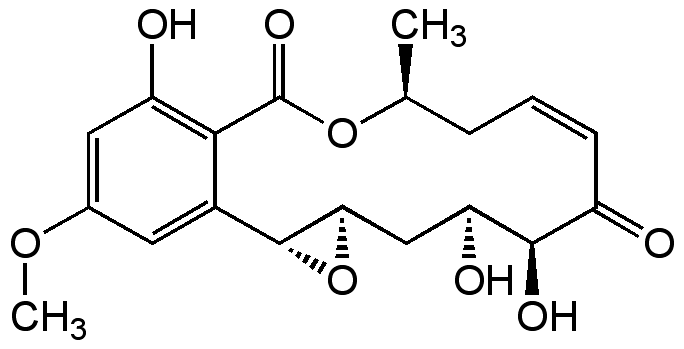
Hypothemycin
Product Code:
BVT-0067
BVT-0067
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0067-C250 | 250 ug | £130.00 |
Quantity:
| BVT-0067-M001 | 1 mg | £365.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Appearance:
White to off-white solid.
CAS:
76958-67-3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H319
InChi:
InChI=1S/C19H22O8/c1-9-4-3-5-12(20)17(23)14(22)8-15-18(27-15)11-6-10(25-2)7-13(21)16(11)19(24)26-9/h3,5-7,9,14-15,17-18,21-23H,4,8H2,1-2H3/b5-3-/t9-,14-,15+,17+,18+/m0/s1
InChiKey:
SSNQAUBBJYCSMY-KNTMUCJRSA-N
Long Description:
Chemical. CAS: 76958-67-3. Formula: C19H22O8. MW: 378.4. Isolated from Phoma sp. Antifungal. Cytotoxic against some tumor cell lines, partly attributed to inhibition of Ras-inducible genes. Inhibits proliferation of mouse and human T cells. Modulates production of cytokines during T cell activation. Facilitates the ubiquitinylation process of cyclin D1. Potent and selective threonine/tyrosine-specific kinase, MEK and other protein kinases inhibitor in both in vitro and in vivo studies.
MDL:
MFCD08457932
Molecular Formula:
C19H22O8
Molecular Weight:
378.4
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P305, P351, P338
Product Description:
Antifungal. Cytotoxic against some tumor cell lines, partly attributed to inhibition of Ras-inducible genes. Inhibits proliferation of mouse and human T cells. Modulates production of cytokines during T cell activation. Facilitates the ubiquitinylation process of cyclin D1. Potent and selective threonine/tyrosine-specific kinase, MEK and other protein kinases inhibitor in both in vitro and in vivo studies.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
COC1=CC(O)=C2C(=C1)[C@H]1O[C@@H]1C[C@H](O)[C@H](O)C(=O)C=C/C[C@H](C)OC2=O
Solubility Chemicals:
Soluble in DMSO or acetone; insoluble in methanol or water.
Source / Host:
Isolated from Phoma sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Metabolites of pyrenomycetes XIII: Structure of (+) hypothemycin, an antibiotic macrolide from hypomyces trichothecoides: M.S.R. Nair & S.T. Carey; Tetrahedron Lett. 21, 2011 (1980) | Metabolites of pyrenomycetes. XIV: Structure and partial stereochemistry of the antibiotic macrolides hypothemycin and dihydrohypothemycin: M.S.R. Nair, et al.; Tetrahedron 37, 2445 (1981) | Revised structure and stereochemistry of hypothemycin: T. Agatsuma et al.; Chem. Pharm. Bull. 41, 373 (1993) | Antitumor efficacy of hypothemycin, a new Ras-signaling inhibitor: H. Tanaka, et al.; Jpn. J. Cancer Res. 90, 1139 (1999) | Hypothemycin inhibits the proliferative response and modulates the production of cytokines during T cell activation: R. Camacho, et al.; Immunopharmacology 44, 255 (1999) | Resorcylic acid lactones: naturally occurring potent and selective inhibitors of MEK: A. Zhao, et al.; J. Antibiot. 52, 1086 (1999) | Suppression of oncogenic transformation by hypothemycin associated with accelerated cyclin D1 degradation through ubiquitin-proteasome pathway: H. Sonoda, et al.; Life Sci. 65, 381 (1999) | Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides: A. Schirmer, et al.; PNAS 103, 4234 (2006) | Chemistry and biology of resorcylic acid lactones: N. Winssinger, et al.; Chem. Commun. (Camb). 1, 22 (2007), (Review) | The resorcyclic acid lactone Hypothemycin selectively inhibits the mitogen-activated protein kinase kinase-extracellular signal-regulated kinase pathway in cells: H. Fukazawa, et al.; Biol. Pharm. Bull. 33, 168 (2010)
Related Products
| Product Name | Product Code | Supplier | Palmarumycin C3 | BVT-0078 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|