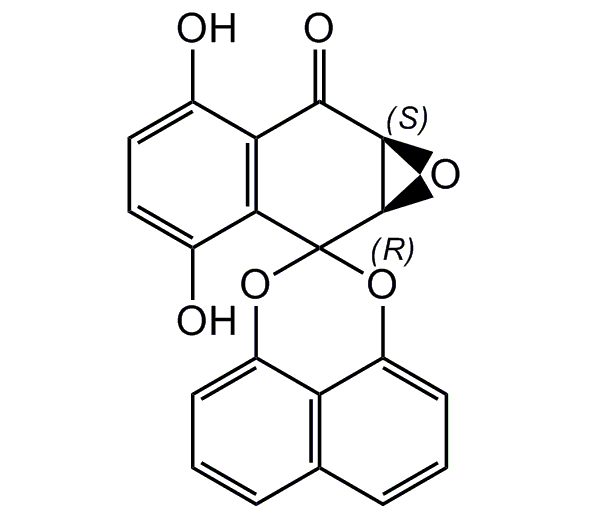
Palmarumycin C3
| Code | Size | Price |
|---|
| BVT-0078-M001 | 1 mg | £150.00 |
Quantity:
| BVT-0078-M005 | 5 mg | £425.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(1a'R,7a'S)-3',6'-Dihydroxy-1a'H-spiro[naphtho[1,8-de][1,3]dioxine-2,2'-naphtho[2,3-b]oxiren]-7'(7a'H)-one
Appearance:
Yellow to green solid.
CAS:
159934-11-9
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319, H330
InChi:
InChI=1S/C20H12O6/c21-10-7-8-11(22)16-15(10)17(23)18-19(24-18)20(16)25-12-5-1-3-9-4-2-6-13(26-20)14(9)12/h1-8,18-19,21-22H/t18-,19-/m1/s1
InChiKey:
BDXBHYOFNNANPN-RTBURBONSA-N
Long Description:
Chemical. CAS: 159934-11-9. Formula: C20H12O6. MW: 348.3. Isolated from Sphaeropsidales sp. Rasfarnesyltransferase inhibitor. Antifungal, antibacterial and herbicidal. Structurally unique natural products. The basic structure of palmarumycin C1 can be modified by a number of hydroxylation, oxygenation, dehydrogenation and chlorination steps. Antioxidant
Molecular Formula:
C20H12O6
Molecular Weight:
348.3
Package Type:
Plastic Vial
PG:
III
Precautions:
P260, P270, P280, P284, P301, P312, P302, P352, P310, P405
Product Description:
Rasfarnesyltransferase inhibitor. Antifungal, antibacterial and herbicidal. Structurally unique natural products. The basic structure of palmarumycin C1 can be modified by a number of hydroxylation, oxygenation, dehydrogenation and chlorination steps. Antioxidant.
Purity:
>97% (HPLC)
Signal word:
Danger
SMILES:
OC1=CC=C(O)C2=C1C(=O)[C@H]1O[C@H]1C21OC2=CC=CC3=C2C(O1)=CC=C3
Solubility Chemicals:
Soluble in DMSO, dichloromethane, acetone or methanol.
Source / Host:
Isolated from Sphaeropsidales sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Biologically Active Metabolites from Fungi, 4. Palmarumycins CP1-CP4 from Coniothyrium palmarum: Isolation, structure elucidation, and biological activity: K. Krohn, et al.; Liebigs Ann. Chem. 1994, 1093 (1994) | Biologically Active Metabolites from Fungi, 5. Palmarumycins C1-C16 from Coniothyrium sp.: Isolation, structure elucidation, and biological activity: K. Krohn, et al.; Liebigs Ann. Chem. 1994, 1099 (1994) | Secondary metabolites by chemical screening, 42 Cladospirones B to I from Sphaeropsidales sp. F-24'707 by variation of culture conditions: H.B. Bode, et al.; Eur. J. Org. Chem. 2000, 3185-3193 (2000) | Unified route to the palmarumycin and preussomerin natural products. Enantioselective synthesis of (-)-preussomerin G: A.G. Barrett, et al.; J. Org. Chem. 67, 2735-2750 (2002) | Natural products derived from naphthalenoid precursors by oxidative dimerization: K. Krohn; Prog. Chem. Org. Nat. Prod. 85, 1-49 (2003) (Review) | Antimicrobial and Antioxidant Activities and Effect of 1-Hexadecene Addition on Palmarumycin C2 and C3 Yields in Liquid Culture of Endophytic Fungus Berkleasmium sp. Dzf12: Y. Mou, et al.; Molecules 18, 15587 (2013)