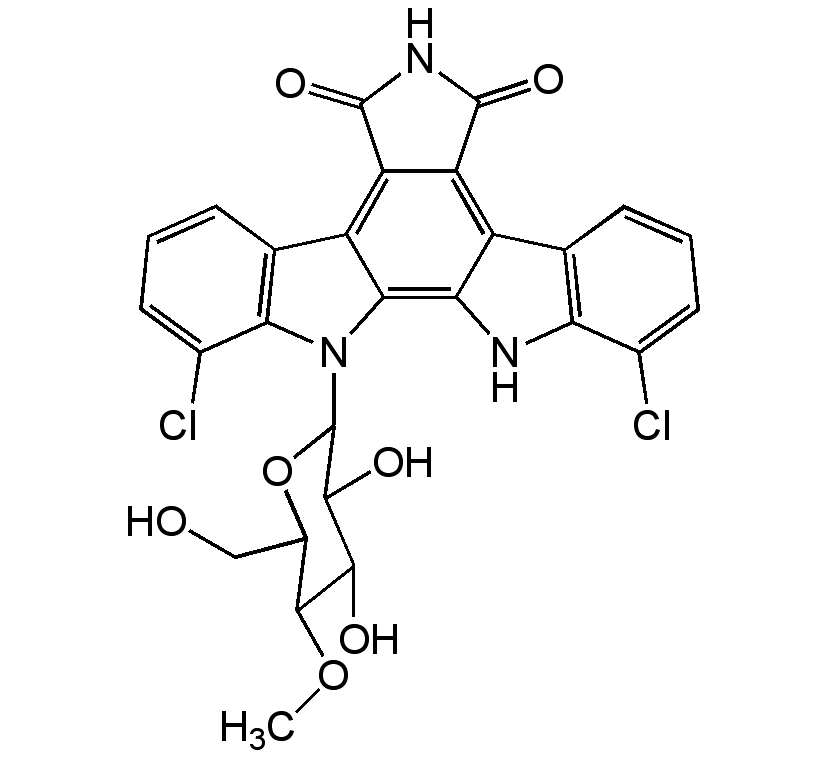
Rebeccamycin
| Code | Size | Price |
|---|
| BVT-0139-C250 | 250 ug | £105.00 |
Quantity:
| BVT-0139-M001 | 1 mg | £300.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 359079; BRN 4732638
Appearance:
Yellow solid.
CAS:
93908-02-2
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H300, H310, H319, H332
InChi:
InChI=1S/C27H21Cl2N3O7/c1-38-24-13(8-33)39-27(23(35)22(24)34)32-20-10(5-3-7-12(20)29)15-17-16(25(36)31-26(17)37)14-9-4-2-6-11(28)18(9)30-19(14)21(15)32/h2-7,13,22-24,27,30,33-35H,8H2,1H3,(H,31,36,37)/t13-,22-,23-,24-,27-/m0/s1
InChiKey:
QEHOIJJIZXRMAN-UMHCKEELSA-N
Long Description:
Chemical. CAS: 93908-02-2. Formula: C27H21Cl2N3O7. MW: 570.4. Isolated from Streptomyces sp. Antibiotic. Weak topoisomerase I (Topo I) inhibitor. Structurally similar to staurosporine. Does not show any inhibitory activity against protein kinases. Shows significant antitumor properties in vitro (IC50 = 480 nM against mouse B16 melanoma cells and IC50 = 500 nM against P388 leukemia cells).
MDL:
MFCD01718312
Molecular Formula:
C27H21Cl2N3O7
Molecular Weight:
570.4
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P262, P280, P301, P310, P302, P350, P312
Product Description:
Antibiotic. Structurally similar to staurosporine. Selective activity against several cancer cell lines. DNA intercalator, resulting in catalytic inhibition of topoisomerases I. Shows significant antitumor properties in vitro (IC50 = 480 nM against mouse B16 melanoma cells and IC50 = 500 nM against P388 leukemia cells). Does not show any inhibitory activity against protein kinases.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
COC1C(O)C(O)C(OC1CO)N1C2=C3NC4=C(C=CC=C4Cl)C3=C3C(=O)NC(=O)C3=C2C2=C1C(Cl)=CC=C2
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C. After reconstitution, prepare aliquots and store at -20°C.
References
Production and biological activity of rebeccamycin, a novel antitumor agent: J.A. Bush, et al.; J. Antibiot. (Tokyo) 40, 668 (1987) | Induction of mammalian DNA topoisomerase I mediated DNA cleavage by antitumor indolocarbazole derivatives: Y. Yamashita, at al.; Biochemisty 31, 12069 (1992) | DNA cleavage by topoisomerase I in the presence of indolocarbazole derivatives of rebeccamycin: C. Bailly, et al.; Biochemistry 36, 3917 (1997) | Syntheses and biological activities (topoisomerase inhibition and antitumor and antimicrobial properties) of rebeccamycin analogues bearing modified sugar moieties and substituted on the imide nitrogen with a methyl group: F. Anizon, et al.; J. Med. Chem. 40, 3456 (1997) | Calories from carbohydrates: energetic contribution of the carbohydrate moiety of rebeccamycin to DNA binding and the effect of its orientation on topoisomerase I inhibition: C. Bailly, et al.; Chem. Biol. 6, 277 (1999) | Discovery of Antitumor Indolocarbazoles: Rebeccamycin, NSC 655649, and Fluoroindolocarbazoles: B.H. Long, et al.; Curr. Med. Chem. 2, 255 (2002) | Rebeccamycin derivatives as dual DNA-damaging agents and potent checkpoint kinase 1 inhibitor: C. Marminon, et al.; Mol Pharmacol 74, 1620-1629 (2008) | Phase II and pharmacokinetic trial of rebeccamycin analog in advanced biliary cancers: A. Dowlati, et al.; Cancer Chemother Pharmacol 65, 73-78 (2009) | New progress of researches in carbazole compounds: F. Zhang, et al.; Chinese J. Org. Chem. 30, 783 (2010) | Checkpoint Kinase 1 activation enhances intestinal epithelial barrier function via regulation of claudin-5 expression: A. Watari, et al.; PLoS One 11, e0145631/1 (2016) | Rebeccamycin attenuates TNF-a-induced intestinal epithelial barrier dysfunction by inhibiting myosin light chain kinase production: A. Watari, et al.; Cell. Physiol. Biochem. 41, 1924 (2017) | The antitumor antibiotic rebeccamycin-challenges and advanced approaches in production processes: K. Pommerehne, et al.; Appl. Microbiol. Biotechnol. 103, 3627 (2019)
Related Products
| Product Name | Product Code | Supplier | Manumycin A | BVT-0091 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|