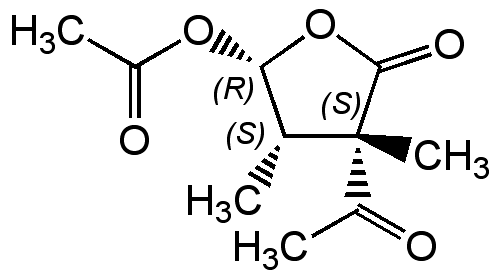
Acetomycin
| Code | Size | Price |
|---|
| BVT-0150-M001 | 1 mg | £110.00 |
Quantity:
| BVT-0150-M005 | 5 mg | £350.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC350598; 3-Acetyl-5-(acetyloxy)dihydro-3,4-dimethyl-2(3H)furanone; (2R,3S,4S)-4-Acetyl-3,4-dimethyl-5-oxotetrahydro-furan-2-yl acetate
Appearance:
White to off-white crystalline solid.
CAS:
510-18-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H319
InChi:
InChI=1S/C10H14O5/c1-5-8(14-7(3)12)15-9(13)10(5,4)6(2)11/h5,8H,1-4H3/t5-,8-,10+/m1/s1
InChiKey:
OYMZTORLGBISLR-RHFNHBFPSA-N
Long Description:
Chemical. CAS: 510-18-9. Formula: C10H14O5. MW: 214.2. Isolated from Streptomyces ramulosus. Antibiotic. Antibacterial, antifungal and antiprotozoal. Cytotoxic against several tumor cell lines in vitro.
MDL:
MFCD01672977
Molecular Formula:
C10H14O5
Molecular Weight:
214.2
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P305, P351, P338
Product Description:
Antibiotic. Antibacterial, antifungal and antiprotozoal. Cytotoxic against several tumor cell lines in vitro.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
C[C@@H]1[C@H](OC(C)=O)OC(=O)[C@]1(C)C(C)=O
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Isolated from Streptomyces ramulosus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
The structure of acetomycin. Spectroscopic characterization and X-ray analysis of a bromo derivative: H. Uhr, et al.; J. Antibiot. 38, 1684 (1985) | Biological effects of acetomycin. I. Activity against tumor cells in vitro and in vivo: S.W. Mamber, et al.; J. Antibiot. 40, 73 (1987) | Biological effects of acetomycin. II. Inactivation by esterases in vitro: S.W. Mamber, et al.; J. Antibiot. 40, 77 (1987) | The structure and absolute configuration of acetomycin: F.H. Cano, et al.; Acta Cryst. C. 44, 919 (1988). | Synthesis and in vitro cytotoxicity of acetomycin and related analogs: D. Chen, et al.; Bioorg. Med. Chem. Lett. 5, 759 (1995) | Total synthesis of (?)-acetomycin and design of esterase-resistant analogs: J. Uenishi, et al.; Chem. Pharm. Bull. 47, 517 (1999) | Formation of chiral quaternary carbon stereocenters using silylene transfer reactions: Enantioselective synthesis of (+)-5-epi-acetomycin: S.A. Calad, et al.; Org. Lett. 9, 1037 (2007)
Related Products
| Product Name | Product Code | Supplier | (+)-Aphidicolin | BVT-0307 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|