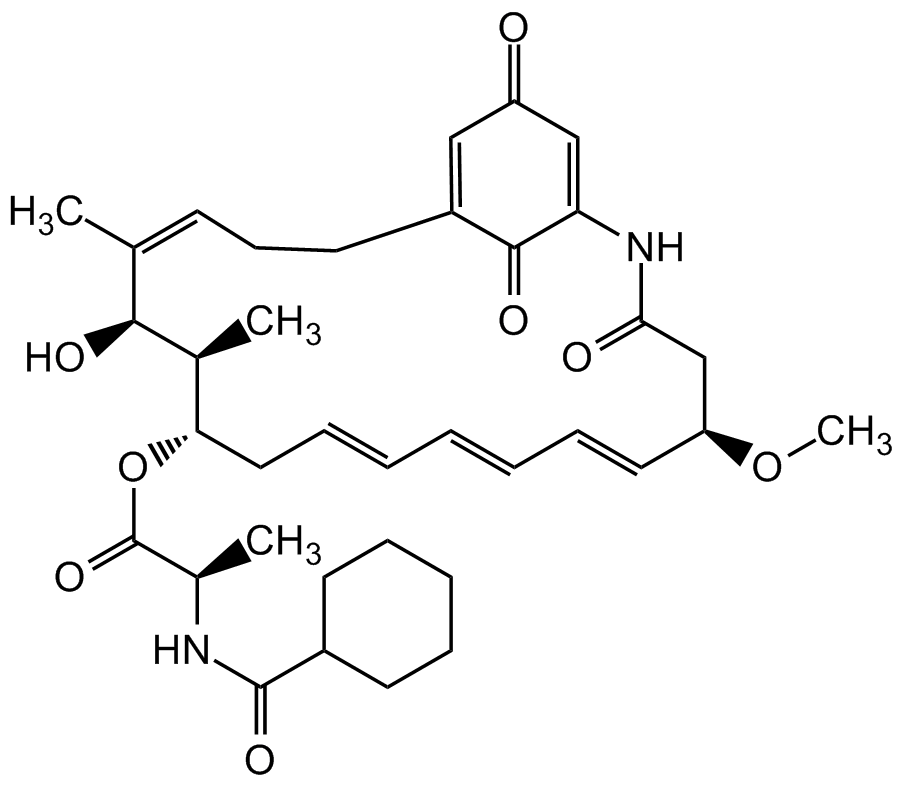
Ansatrienin A
| Code | Size | Price |
|---|
| BVT-0246-M001 | 1 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Mycotrienin I; Antibiotic T 23I
Appearance:
Yellow powder.
CAS:
82189-03-5
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H301, H311, H319, H332
InChi:
InChI=1S/C36H48N2O8/c1-23-14-13-17-27-20-28(39)21-30(34(27)42)38-32(40)22-29(45-4)18-11-6-5-7-12-19-31(24(2)33(23)41)46-36(44)25(3)37-35(43)26-15-9-8-10-16-26/h5-7,11-12,14,18,20-21,24-26,29,31,33,41H,8-10,13,15-17,19,22H2,1-4H3,(H,37,43)(H,38,40)/b6-5+,12-7+,18-11+,23-14-/t24-,25+,29-,31-,33-/m0/s1
InChiKey:
WWUVMHRJRCRFSL-UOZMSBJPSA-N
Long Description:
Chemical. CAS: 82189-03-5. Formula: C36H48N2O8. MW: 636.8. Isolated from Streptomyces collinus. Antibiotic. Antitumor compound. Antifungal. Inhibits osteoclastic bone resorption.
MDL:
MFCD01674354
Molecular Formula:
C36H48N2O8
Molecular Weight:
636.8
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P280, P301, P310, P302, P352, P312, P405
Product Description:
Antibiotic. Antitumor compound. Active against several cell lines. Shown to potentiate several clinical anti-cancer agents. Inhibits TNF-alpha-induced expression of ICAM-1 (IC50=570nM). Antifungal. Inhibits osteoclastic bone resorption.
Purity:
>98% (HPLC)
Signal Word:
Danger
SMILES:
CO[C@@H]1CC(=O)NC2=CC(=O)C=C(CCC=C(C)/[C@H](O)[C@@H](C)[C@H](CC=CC=CC=C1)OC(=O)[C@@H](C)NC(=O)C1CCCCC1)C2=O
Solubility Chemicals:
Soluble in 100% ethanol, methanol, dimethyl formamide or DMSO.
Source / Host:
Isolated from Streptomyces collinus.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Mycotrienin, a new polyene antibiotic isolated from Streptomyces: C. Coronelli, et al.; J. Antibiot. (Tokyo) 20, 329 (1967) | Die Konstitution der fungistatischen Ansamycin-Antibiotica Ansatrienin A und B: M. Damberg, et al.; Tetrahedron Lett. 23, 59 (1982) | Studies on mycotrienin antibiotics, a novel class of ansamycins. I. Taxonomy, fermentation, isolation and properties of mycotrienins I and II: M. Sugita, et al.; J. Antibiot. (Tokyo) 35, 1460 (1982) | Studies on mycotrienin antibiotics, a novel class of ansamycins. II. Structure elucidation and biosynthesis of mycotrienins I and II: M. Sugita, et al.; J. Antibiot. (Tokyo) 35, 1467 (1982) | Potentiation of mitomycin C, 6-mercaptopurine, bleomycin, cis-diamminedichloroplatinum and 5-fluorouracil by mycotrienins and mycotrienols: M. Kuwano, et al.; Gann. 74, 759 (1983) | Mycotrienins. A new class of potent inhibitors of osteoclastic bone resorption: D. Feuerbach, et al.; J. Biol. Chem. 270, 25949 (1995) | (+)-trienomycin A, B, C, and F and (+)-mycotrienins I and II: relative and absolute stereochemistry: A. B. Smith, et al.; JACS 118, 8308 (1996) | Total syntheses of (+)-trienomycins A and F via a unified strategy: A. B. Smith, et al.; JACS 118, 8316 (1996) | Total synthesis of (+)-mycotrienol and (+)-mycotrienin I: application of asymmetric crotylsilane bond constructions: C. E. Masse, et al.; JACS 120, 4123-4134 (1998) | Mycotrienin II, a translation inhibitor that prevents ICAM-1 expression induced by pro-inflammatory cytokines: Y. Yamada, et al.; J. Antibiot. 64, 361 (2011)