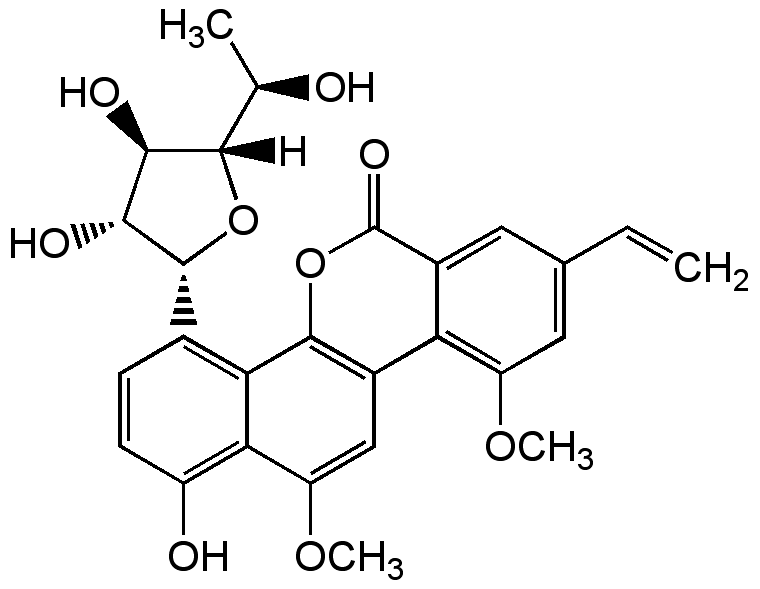
Gilvocarcin V
Product Code:
BVT-0256
BVT-0256
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0256-C100 | 100 ug | £90.00 |
Quantity:
| BVT-0256-C250 | 250 ug | £140.00 |
Quantity:
| BVT-0256-M001 | 1 mg | £340.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Anandimycin A; Toromycin A; NSC 338943; DC-38-V; Antibiotic 1072B
Appearance:
Yellow solid.
CAS:
77879-90-4
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H300, H310, H319, H330, H351
InChi:
InChI=1S/C27H26O9/c1-5-12-8-15-19(17(9-12)33-3)14-10-18(34-4)21-16(29)7-6-13(20(21)25(14)36-27(15)32)26-23(31)22(30)24(35-26)11(2)28/h5-11,22-24,26,28-31H,1H2,2-4H3/t11-,22-,23-,24+,26-/m1/s1
InChiKey:
XCWHINLKQMCRON-UCDARZNSSA-N
Long Description:
Chemical. CAS: 77879-90-4. Formula: C27H26O9. MW: 494.5. Isolated from Streptomyces sp. G? 3592. Antibiotic. Antitumor compound. Weakly active against Gram-positive bacteria and fungi. Mediates a unique cross-linking reaction between DNA and histone H3 by light. Single strand scission and covalent binding to DNA after photoactivation.
MDL:
MFCD01939442
Molecular Formula:
C27H26O9
Molecular Weight:
494.5
Package Type:
Plastic Vial
PG:
III
Precautions:
P201, P260, P262, P280, P284, P301, P310, P302, P350, P310, P405
Product Description:
Antibiotic. Antitumor compound. Weakly active against Gram-positive bacteria and fungi. Mediates a unique cross-linking reaction between DNA and histone H3 by light. Single strand scission and covalent binding to DNA after photoactivation.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@]1(O[C@@H]([C@H](O)[C@H]1O)C1=C2C3=C(C=C(OC)C2=C(O)C=C1)C1=C(OC)C=C(C=C)C=C1C(=O)O3)[C@@H](C)O
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Isolated from Streptomyces sp. G? 3592.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C.
Documents
References
Gilvocarcins, new antitumor antibiotics. 1. Taxonomy, fermentation, isolation and biological activities: H. Nakano, et al.; J. Antibiot. (Tokyo) 34, 266 (1981) | Activation of antitumor agent gilvocarcins by visible light: R.K. Elespuru and S.K. Gonda; Science 223, 69 (1984) | Photosensitized DNA breaks and DNA-to-protein crosslinks induced in human cells by antitumor agent gilvocarcin V: M.J. Peak, et al.; Chem. Biol. Interact. 67, 267 (1988) | Biochemical characterisation of elsamicin and other coumarin-related antitumour agents as potent inhibitors of human topoisomerase II: A. Lorico & B. H. Long; Eur. J. Cancer 29A, 1985 (1993) | Photophysical properties of gilvocarcins V and M and their binding constant to calf thymus DNA: R. Oyola, et al.; Photochem. Photobiol. 65, 802 (1997) | Histone H3 and heat shock protein GRP78 are selectively cross-linked to DNA by photoactivatedgilvocarcin V in human fibroblasts: A. Matsumoto and P.C. Hanawalt; Cancer Res. 60, 3921 (2000) | The complete gene cluster of the antitumor agent gilvocarcin V and its implication for the biosynthesis of the gilvocarcins: C. Fischer, et al.; J. Am. Chem. Soc. 125, 7818-7819 (2003) | Multi-oxygenase complexes of the gilvocarcin and jadomycin biosyntheses: M. K. Kharel, et al.; J. Am. Chem. Soc. 129, 3780-3781 (2007) | Inactivation of the ketoreductase gilU gene of the gilvocarcin biosynthetic gene cluster yields new analogues with partly improved biological activity: T. Liu, et al.; Chem Bio Chem 10, 278-286 (2009) | Total synthesis of the antitumor natural product polycarcin V and evaluation of its DNA binding profile: X. Cai, et al.; Org. Lett. 16, 2962 (2014)