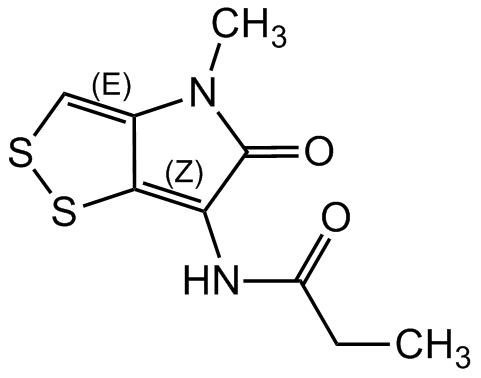
Aureothricin
| Code | Size | Price |
|---|
| BVT-0345-C500 | 500 ug | £55.00 |
Quantity:
| BVT-0345-M001 | 1 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Propionylpyrrothione; Farcinicin; N-(4-Methyl-5-oxo-4,5-dihydro-[1,2]dithiolo[4,3-b]pyrrol-6-yl)propionamide
Appearance:
Yellow powder.
CAS:
574-95-8
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Hazards:
H300, H310, H311, H319, H332
InChi:
InChI=1S/C9H10N2O2S2/c1-3-6(12)10-7-8-5(4-14-15-8)11(2)9(7)13/h4H,3H2,1-2H3,(H,10,12)
InChiKey:
UGZYFXMSMFMTSM-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 574-95-8. Formula: C9H10N2O2S2. MW: 242.3. Isolated from Streptomyces thioluteus. Antibiotic. Active against Gram-positive and Gram-negative bacteria, yeast, filamentous fungi, protozoa and insects. Potent bacterial and yeast RNA polymerases inhibitor. Inhibitor of fungal mannan and glucan formation. Similar to thiolutin. Antitumor compound.
MDL:
MFCD07370147
Molecular Formula:
C9H10N2O2S2
Molecular Weight:
242.3
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P262, P280, P301, P310, P302, P350, P310, P405
Product Description:
Antibiotic. Active against Gram-positive and Gram-negative bacteria, yeast, filamentous fungi, protozoa and insects. Potent bacterial and yeast RNA polymerases inhibitor. Inhibitor of fungal mannan and glucan formation. Similar to thiolutin. Antitumor compound.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
CCC(=O)NC1=C2SSC=C2N(C)C1=O
Solubility Chemicals:
Soluble in DMSO or dimethyl formamide; partially soluble in methanol or 100% ethanol; poorly soluble in water.
Source / Host:
Isolated from Streptomyces thioluteus.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
The structures of thiolutin and aureothricin, antibiotics containing a unique pyrrolinonodithiole nucleus: W. D. Celmer & I. A. Solomons; J. Am. Chem. Soc. 77, 2861 (1955) | Anticancer properties of dithiolopyrrolones: J.M. Webster, et al.; US Patent 6,020,360, (2000) | Thiolutin, an inhibitor of huvec adhesion to vitronectin, reduces paxillin in huvecs and suppresses tumor cell-induced angiogenesis: K. Minamiguchi et al.; Int. J. Cancer 93, 307 (2001) | Expedient total synthesis of pyrrothine natural products and analogs: T. Hjelmgaard et al.; Org. Biomol. Chem. 5, 344 (2007) | Dithiolopyrrolone antibiotic formation induced by adding valeric acid to the culture broth of Saccharothrix algeriensis: R. Merrouche et al.; J. Nat. Prod. 73, 1164 (2010)
Related Products
| Product Name | Product Code | Supplier | (S)-(+)-Ascochin | BVT-0309 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|