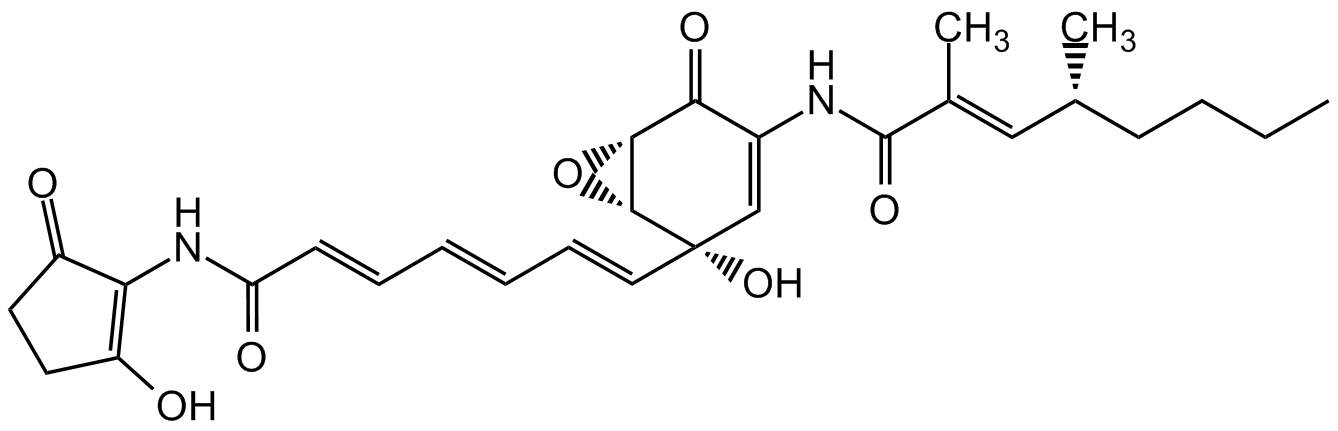
Manumycin B
| Code | Size | Price |
|---|
| BVT-0264-M001 | 1 mg | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
UCF1A; N98-1272A
Appearance:
Yellow to brown powder.
CAS:
139023-58-8
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
After reconstitution protect from light at -20°C.Protect from light.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C28H34N2O7/c1-4-5-10-17(2)15-18(3)27(35)29-19-16-28(36,26-25(37-26)24(19)34)14-9-7-6-8-11-22(33)30-23-20(31)12-13-21(23)32/h6-9,11,14-17,25-26,31,36H,4-5,10,12-13H2,1-3H3,(H,29,35)(H,30,33)/b7-6+,11-8+,14-9+,18-15+/t17-,25-,26-,28+/m1/s1
InChiKey:
ZGICGDCGECBVTD-LOWJZYKPSA-N
Long Description:
Chemical. CAS: 139023-58-8. Formula: C28H34N2O7. MW: 510.6. Isolated from Streptomyces parvulus. Antibiotic. Antibacterial. Active against Gram-positive bacteria. Rasfarnesyltransferase inhibitor. Apoptosis (Caspase-1) inhibitor. AChE inhibitor.
MDL:
MFCD00920784
Molecular Formula:
C28H34N2O7
Molecular Weight:
510.6
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic. Antibacterial. Active against Gram-positive bacteria. Rasfarnesyltransferase inhibitor. Apoptosis (Caspase-1) inhibitor. AChE inhibitor.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CCCC[C@@H](C)C=C(/C)C(=O)NC1=C[C@@](O)(C=CC=CC=CC(=O)NC2=C(O)CCC2=O)[C@@H]2O[C@@H]2C1=O
Solubility Chemicals:
Soluble in DMSO, methanol or chloroform.
Source / Host:
Isolated from Streptomyces parvulus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
New compounds of the manumycin group of antibiotics and a facilitated route for their structure elucidation: I. Sattler, et al.; J. Org. Chem. 58, 6583 (1993) | Identification of ras farnesyltransferase inhibitors by microbial screening: M. Hara, et al.; PNAS 90, 2281 (1993) | TMC-1 A, B, C and D, new antibiotics of the Manumycin group produced by Streptomyces sp.: J. Kohno, et al.; J. Antibiot. 49, 1212 (1996) | EI-1511-3, -5 and EI-1625-2, novel interleukin-1 beta converting enzyme inhibitors produced by Streptomyces sp. E-1511 and E-1625. III. Biochemical properties of EI-1511-3, -5 and EI-1625-2: T. Tanaka, et al.; J. Antibiot. 49, 1085 (1996) | The manumycin-group metabolites: I. Sattler, et al.; Nat. Prod. Rep. 15, 221 (1998) (Review) | The first total synthesis of a type II manumycin antibiotic, (+)-TMC-1 A: the total syntheses of (-)-LL-C10037beta and(+)-manumycin B: J.J. Cronj? Grov?, et al.; Chem. Commun. 1999, 421 (1999) | Isolation and characterization of N98-1272 A, B and C, selective acetylcholinesterase inhibitors from metabolites ofan actinomycete strain: Z.-H. Zheng, et al.; J. Enzyme Inh. Med. Chem. 22, 43 (2007)