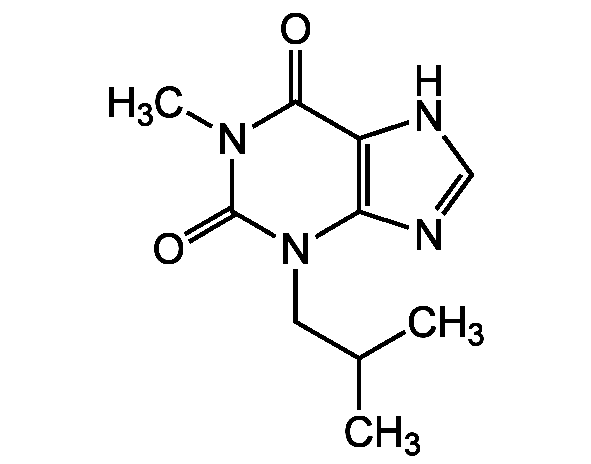
IBMX
Product Code:
AG-CR1-3512
AG-CR1-3512
Regulatory Status:
RUO
RUO
Shipping:
-20°C
-20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CR1-3512-M500 | 500 mg | £95.00 |
Quantity:
| AG-CR1-3512-G001 | 1 g | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
3-Isobutyl 1-methylxanthine; NSC 165960; EINECS 249-259-3
Appearance:
White to off-white solid.
CAS:
28822-58-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302
InChi:
InChI=1S/C10H14N4O2/c1-6(2)4-14-8-7(11-5-12-8)9(15)13(3)10(14)16/h5-6H,4H2,1-3H3,(H,11,12)
InChiKey:
APIXJSLKIYYUKG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 28822-58-4. Formula: C10H14N4O2. MW: 222.3. Cell permeable, competitive, non-specific cAMP and cGMP phosphodiesterase inhibitor. Increases cAMP levels that activate PKA, leading to decreased proliferation, increased differentiation and induction of apoptosis. Enhances differentiation of 3T3-L1 cells. Non-selective adenosine receptor antagonist. Inhibits Ca2+ ion channels. Activates TNF-alpha. Adipogenic. Activates leukotriene synthesis. Reduces inflammation and innate immunity.
MDL:
MFCD00005584
Molecular Formula:
C10H14N4O2
Molecular Weight:
222.3
Package Type:
Vial
Precautions:
P270, P301, P312, P330
Product Description:
Cell permeable, competitive, non-specific cAMP and cGMP phosphodiesterase inhibitor [1, 5, 6, 11]. Increases cAMP levels that activate PKA, leading to decreased proliferation, increased differentiation and induction of apoptosis [9]. Enhances differentiation of 3T3-L1 cells [3]. Non-selective adenosine receptor antagonist [4]. Inhibits Ca2+ ion channels [7, 8]. Activates TNF-alpha [10]. Adipogenic [10]. Activates leukotriene synthesis [12]. Reduces inflammation and innate immunity [12].
Purity:
>99% (HPLC)
Signal word:
Warning
SMILES:
CC(C)CN1C2=C(NC=N2)C(=O)N(C)C1=O
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol. Insoluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Effects of xanthine derivatives on lipolysis and on adenosine 3',5'- monophosphate phosphodiesterase activity: J.A. Beavo, et al.; Mol. Pharmacol. 6, 597 (1970) | Inhibition of growth of primary and metastatic Lewis lung carcinoma cells by the phosphodiesterase inhibitor isobutylmethylxanthine: P. Janik, et al.; Cancer Res. 40, 1950 (1980) | A role for soluble cAMP phosphodiesterases in differentiation of 3T3-L1adipocytes: M.L. Elks & V.C. Manganiello; J. Cell Physiol. 124, 191 (1985) | Adenosine receptors: development of selective agonists and antagonists: J.W. Daly, et al.; Prog. Clin. Biol. Res. 230, 41 (1987) | Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors: J.A. Beavo & D.H. Reifsnyder; TIPS 11, 150 (1990) | Isobutylmethylxanthine and other classical cyclic nucleotide phosphodiesterase inhibitors affect cAMP-dependent protein kinase activity: C. Tomes, et al.; Cell Signal. 5, 615 (1993) | IBMX induces calcium release from intracellular stores in rat sensory neurones: Y. Usachev & A. Verkhratsky; Cell Calcium 17, 197 (1995) | Inhibition of recombinant human cardiac L-type Ca2+ channel alpha1C subunits by 3-isobutyl-1-methylxanthine: I.M. Fearon, et al.; Eur. J. Pharmacol. 342, 353 (1998) | Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas: T.C. Chen, et al.; Lab. Invest. 78, 165 (1998) | The phosphodiesterase inhibitor IBMX suppresses TNF-alpha expression in human adipocyte precursor cells: a possible explanation for its adipogenic effect: F. Hube, et al.; Horm. Metab. Res. 31, 359 (1999) | Cyclic nucleotide phosphodiesterases: D.M. Essayan; J. Allergy Clin. Immunol. 108, 671 (2001) | Leukotrienes: underappreciated mediators of innate immune responses: M. Peters-Golden, et al.; J. Immunol. 174, 589 (2005)
Related Products
| Product Name | Product Code | Supplier | Papaverine . hydrochloride | AG-CN2-0414 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|