GYY 4137
| Code | Size | Price |
|---|
| AG-CR1-3513-M010 | 10 mg | £60.00 |
Quantity:
| AG-CR1-3513-M050 | 50 mg | £210.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
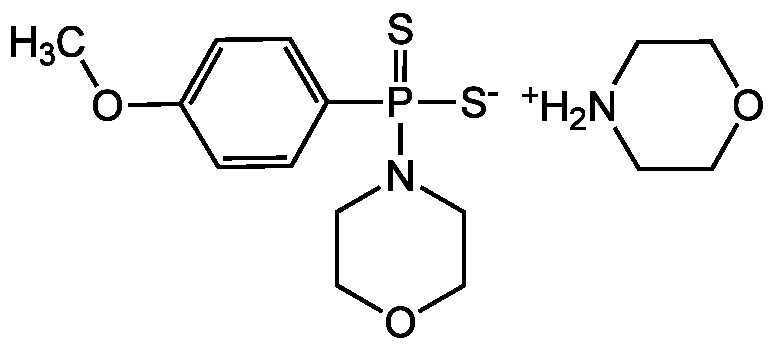
4-Methoxyphenyl(morpholino)phosphinodithioate morpholinium salt
Appearance:
White solid.
CAS:
106740-09-4
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C11H16NO2PS2.C4H9NO/c1-13-10-2-4-11(5-3-10)15(16,17)12-6-8-14-9-7-12;1-3-6-4-2-5-1/h2-5H,6-9H2,1H3,(H,16,17);5H,1-4H2
InChiKey:
YZMHNNLDUWRZFW-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 106740-09-4. Formula: C11H16NO2PS2 . C4H9NO. MW: 288.4 . 87.1. Water-soluble, slow-releasing hydrogen sulfide (H2S) donor. Exhibits vasodilator and antihypertensive activity. Anti-inflammatory. Inhibits LPS-induced release of proinflammatory mediators (IL-1beta, IL-6, TNF-alpha, nitric oxide (NO) and PGE2) and increases the synthesis of the anti-inflammatory chemokine IL-10 through NF-kappaB, ATF-2 and HSP27 dependent pathways. Causes stomatal opening and reduces nitric oxide accumulation in plants. Oxidative stress-induced cell death inhibitor. Shows novel anti-cancer effects in vitro and in vivo. Anti-thrombotic via p-selectin dependent mechanism.
MDL:
MFCD18428020
Molecular Formula:
C11H16NO2PS2 . C4H9NO
Molecular Weight:
288.4 . 87.1
Package Type:
Vial
Product Description:
Water-soluble, slow-releasing hydrogen sulfide (H2S) donor [1]. Exhibits vasodilator and antihypertensive activity [1, 2, 4]. Anti-inflammatory [2, 3]. Inhibits LPS-induced release of proinflammatory mediators (IL-1beta, IL-6, TNF-alpha, nitric oxide (NO) and PGE2) and increases the synthesis of the anti-inflammatory chemokine IL-10 through NF-kappaB, ATF-2 and HSP27 dependent pathways [2, 3]. Causes stomatal opening and reduces nitric oxide accumulation in plants [5]. Oxidative stress-induced cell death inhibitor [6]. Shows novel anti-cancer effects in vitro and in vivo [7]. Anti-thrombotic via p-selectin dependent mechanism [8].
Purity:
>95% (NMR)
SMILES:
C1COCC[NH2+]1.COC1=CC=C(C=C1)P([S-])(=S)N1CCOCC1
Solubility Chemicals:
Soluble in DMSO or water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide.: L. Li et, al.; Circulation 117, 2351 (2008) | GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat.: L. Li, et al.; Free Radic. Biol. Med. 47, 103 (2009) | The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages: M. Whiteman, et al.; Antioxid. Redox Signal. 12, 1147 (2010) | Hydrogen sulfide and its modulation in arterial hypertension and atherosclerosis: J. Be?towski, et al.; Cardiovasc. Hematol. Agents Med. Chem. 8, 173 (2010) | A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation: M. Lisjak, et al.; Plant Physiol. Biochem. 48, 931 (2010) | Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is h(2) S a novel cytoprotective mediator in the inflamed joint?: B. Fox, et al.; J. Cell. Mol. Med. 16, 896 (2012) | The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo: Z.W. Lee, et al.; PLOS One 6, e21077 (2011) | The slow releasing H2S donor GYY 4137 exerts anti-thrombotic effects via a P-selectin dependent mechanism: E. Grambow, et al.; DGCH M?nchen (Meeting Abstract) (2011)
Related Products
| Product Name | Product Code | Supplier | Thioglycine | AG-CR1-3530 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|