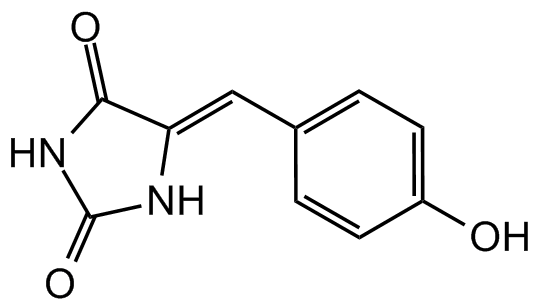
Phenylmethylene hydantoin
Product Code: AG-CN2-0041
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0041-M001 | 1 mg | £155.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
PMH; 5(4-Hydroxybenzylidene)hydantoin
Appearance:
Greenish solid.
CAS:
80171-33-1
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.Protect from light.
InChi:
InChI=1S/C10H8N2O3/c13-7-3-1-6(2-4-7)5-8-9(14)12-10(15)11-8/h1-5,13H,(H2,11,12,14,15)/b8-5-
InChiKey:
UPDDIBZITPTASO-YVMONPNESA-N
Long Description:
Chemical. CAS: 80171-33-1. Formula: C10H8N2O3. MW: 204.2. Isolated from an unidentifed marine sponge. Anticancer compound. Chemopreventive. Shows anti-metastatic activity in prostate cancer cells through enhancement of cell-cell adhesion. Anti-invasive compound. Glycogen synthase kinase-3beta (GSK-3 beta) inhibitor.
MDL:
MFCD00117855
Molecular Formula:
C10H8N2O3
Molecular Weight:
204.2
Package Type:
Vial
Product Description:
Anticancer compound [2, 3, 5, 6]. Chemopreventive [2, 3, 5, 6, 7]. Shows anti-metastatic activity in prostate cancer cells through enhancement of cell-cell adhesion [2, 3]. Anti-invasive compound [5, 6, 7]. Glycogen synthase kinase-3beta (GSK-3 beta) inhibitor [4].
Purity:
>97% (HPLC)
SMILES:
OC1=CC=C(C=C2/NC(=O)NC2=O)C=C1
Solubility Chemicals:
Soluble in ethanol or DMSO.
Source / Host:
Isolated from an unidentifed marine sponge.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
The synthesis of amino-acids: Piperidine and diethylamine as catalysts in the condensation of aromatic aldehydes with hydantoins: W.J. Boyd & W. Robson; Biochem. J. 29, 542 (1935) | Identification of a small molecule class to enhance cell-cell adhesion and attenuate prostate tumor growth and metastasis: G.V. Shah, et al.; Mol. Cancer Ther. 8, 509 (2009) | Discovery, design, and synthesis of anti-metastatic lead phenylmethylene hydantoins inspired by marine natural products: M. Mudit, et al.; Bioorg. Med. Chem. 17, 1731 (2009) | The marine natural-derived inhibitors of glycogen synthase kinase-3beta phenylmethylene hydantoins: In vitro and in vivo activities and pharmacophore modeling: M.A. Khanfar, et al.; Bioorg. Med. Chem. 17, 6032 (2009) | Phenylmethylene hydantoins as prostate cancer invasion and migration inhibitors. CoMFA approach and QSAR analysis: M.A. Khanfar & K.A. El Sayed; Eur. J. Med. Chem. 45, 5397 (2010) | Phenyl-methylene hydantoins alter CD44-specific ligand binding of benign and malignant prostate cells and suppress CD44 isoform expression: K. Yang, et al.; Am. J. Transl. Res. 2, 88 (2010) | Methods for evaluation of structural and biological properties of antiinvasive natural products: M. Mudit, et al.; Meth. Mol. Biol. 716, 55 (2011)