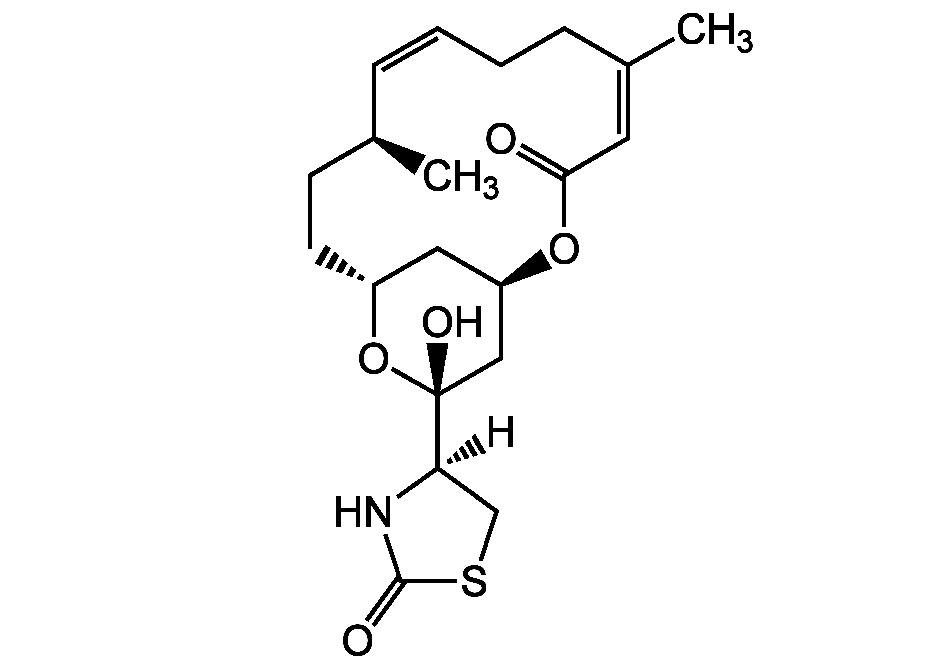
16-epi-Latrunculin B
Product Code: AG-CN2-0034
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0034-C100 | 100 ug | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
16-epi-LatB
Appearance:
Colorless viscous film.
CAS:
444911-05-1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Hazards:
H302, H312, H332
InChi:
InChI=1S/C20H29NO5S/c1-13-5-3-4-6-14(2)9-18(22)25-16-10-15(8-7-13)26-20(24,11-16)17-12-27-19(23)21-17/h3,5,9,13,15-17,24H,4,6-8,10-12H2,1-2H3,(H,21,23)/b5-3-,14-9-/t13-,15-,16-,17-,20-/m1/s1
InChiKey:
NSHPHXHGRHSMIK-WAXGXQFOSA-N
Long Description:
Chemical. CAS: 444911-05-1. Formula: C20H29NO5S. MW: 395.5. Isolated from marine sponge Negombata magnifica. Antiviral (against herpes simplex type 1 virus (HSV-1)). Cytotoxic. Depolymerizes actin filaments (F-actin).
MDL:
MFCD28898633
Molecular Formula:
C20H29NO5S
Molecular Weight:
395.5
Package Type:
Vial
Precautions:
P261, P280, P301, P312, P302, P352, P304, P340
Product Description:
Antiviral (against herpes simplex type 1 virus (HSV-1)) [1]. Cytotoxic [1, 5]. Depolymerizes actin filaments (F-actin) [2, 3, 4].
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
[H][C@@]1(CSC(=O)N1)[C@@]1(O)C[C@H]2C[C@@H](CC[C@H](C)C=C/CCC(C)=C/C(=O)O2)O1
Solubility Chemicals:
Soluble in DMSO or ethanol.
Source / Host:
Isolated from marine sponge Negombata magnifica.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Toward computing relative configurations: 16-epi-latrunculin B, a new stereoisomer of the actin polymerization inhibitor latrunculin B: T.R. Hoye, et al.; JACS 124, 7405 (2002) Abstract | A new dimension to the biosynthetic products isolated from the sponge Negombata magnifica: B. Vilozny, et al.; J. Nat. Prod. 67, 1055 (2004) | Diverted total synthesis: Preparation of a focused library of latrunculin analogues and evaluation of their actin-binding properties: A. F?rstner, et al.; PNAS 102, 8103 (2005) | Total syntheses of the actin-binding macrolides latrunculin A, B, C, M, S and 16-epi-latrunculin B: A. F?rstner, et al.; Chemistry 13, 115 (2007) | Interrogating the Bioactive Pharmacophore of the Latrunculin Chemotype by Investigating the Metabolites of Two Taxonomically Unrelated Sponges: T. Amagata, et al.; J. Med. Chem. 51, 7234 (2008) | Differential binding of Latrunculins to G?Actin: A molecular dynamics study: M. A. Helal, et al. J. Chem. Inf. Model. 53, 2369 (2013) | Marine Drugs: A hidden wealth and a new epoch for cancer management: S. Eram, et al.; Curr. Drug. Metab. 19, (2018)