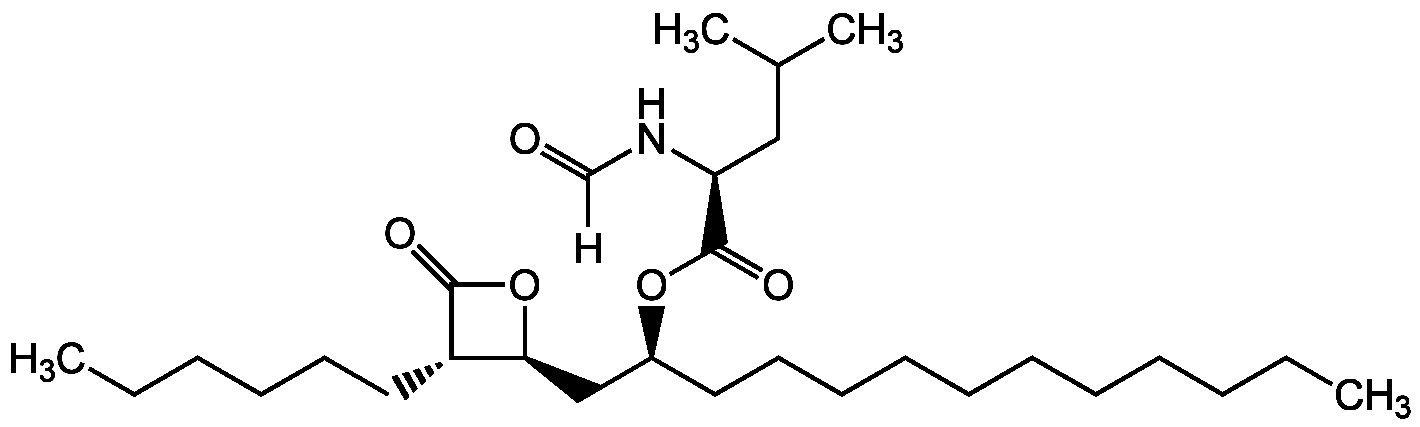
Orlistat
Product Code: AG-CN2-0050
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0050-M050 | 50 mg | £35.00 |
Quantity:
| AG-CN2-0050-M250 | 250 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
Tetrahydrolipstatin; Xenical; Alli; Ro-18-0647
Appearance:
White to off-white solid.
CAS:
96829-58-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
After reconstitution, prepare aliquots and store at -20°C.Protect from light and moisture.
InChi:
InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1
InChiKey:
AHLBNYSZXLDEJQ-FWEHEUNISA-N
Long Description:
Chemical. CAS: 96829-58-2. Formula: C29H53NO5. MW: 495.7. Synthetic. Originally isolated from Streptomyces sp. Hypolipemic cell permeable and irreversible pancreatic, gastric and carboxylester lipase inhibitor. Anti-obesity and antihypercholesterolemic compound. Antitumor compound by inhibition of the thioesterase domain of fatty acid synthase (FASN). Anti-proliferative. Causes cell cycle arrest at G1 phase. Apoptosis inducer through caspase-3 activation. Sn-1-selective-diacylglycerol lipases alpha (DAGLalpha) inhibitor. Targets serine hydrolases in the nervous system, such as diacylglycerol lipase (DAGL), which is responsible for the conversion of DAG to 2-AG. Partially inhibits the hydrolysis of triglycerides and lowers the absorption of dietary fat, promoting weight loss. Promotes the sensitivity to TRAIL in cancer cells by ROS-mediated pathways.
MDL:
MFCD05662360
Molecular Formula:
C29H53NO5
Molecular Weight:
495.7
Package Type:
Vial
Product Description:
Hypolipemic cell permeable and irreversible pancreatic, gastric and carboxylester lipase inhibitor [1-3]. Anti-obesity and antihypercholesterolemic compound [2, 5, 11]. Antitumor compound by inhibition of the thioesterase domain of fatty acid synthase (FASN) [4, 6, 9, 10]. Anti-proliferative [4, 6, 9, 10]. Causes cell cycle arrest at G1 phase. Apoptosis inducer through caspase-3 activation [6, 10]. Sn-1-selective-diacylglycerol lipases alpha (DAGLalpha) inhibitor. Targets serine hydrolases in the nervous system, such as diacylglycerol lipase (DAGL), which is responsible for the conversion of DAG to 2-AG [7]. Partially inhibits the hydrolysis of triglycerides and lowers the absorption of dietary fat, promoting weight loss [8]. Promotes the sensitivity to TRAIL in cancer cells by ROS-mediated pathways [11].
Purity:
>98% (NMR)
SMILES:
[H]C(=O)N[C@@H](CC(C)C)C(=O)O[C@@H](CCCCCCCCCCC)C[C@@H]1OC(=O)[C@H]1CCCCCC
Solubility Chemicals:
Soluble in DMSO, ethanol or DMF.
Source / Host:
Synthetic. Originally isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Interactions of lipoprotein lipase with the active-site inhibitor tetrahydrolipstatin (Orlistat): A. Lookene, et al.; Eur. J. Biochem. 222, 395 (1994) | Mode of action of orlistat: R. Guerciolini; Int. J Obes. Relat. Metab. Disord. 2, S12 (1997) (Review) | Degree of in vivo inhibition of human gastric and pancreatic lipases by Orlistat (Tetrahydrolipstatin, THL) in the stomach and small intestine: B. Sternby, et al.; Clin. Nutr. 21, 395 (2002) | Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity: S.J. Kridel, et al.; Cancer Res. 64, 2070 (2004) | The use of orlistat in the treatment of obesity, dyslipidaemia and Type 2 diabetes: R.H. Nelson & J.M. Miles; Expert Opin. Pharmacother. 6, 2483 (2005) (Review) | Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene: J.A. Menendez, et al.; Ann. Oncol. 16, 1253 (2005) | Development of the first potent and specific inhibitors of endocannabinoid biosynthesis: T. Bisogno, et al.; Biochim. Biophys. Acta 1761, 205 (2006) | Orlistat accelerates gastric emptying and attenuates GIP release in healthy subjects: F.Y. Enc, et al.; Am. J. Physiol. Gastrointest. Liver Physiol. 296, G482 (2008) | Fatty acid synthase inhibition with Orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model: M.A. Carvalho, et al.; Int. J. Cancer. 123, 2557 (2008) | Antitumor effect of orlistat, a fatty acid synthase inhibitor, is via activation of caspase-3 on human colorectal carcinoma-bearing animal: H.Y. Chuang, et al.; Biomed. Pharmacother. 65, 286 (2011) | The anti-obesity drug orlistat promotes sensitivity to TRAIL by two different pathways in hormone-refractory prostate cancer cells: J. Fujiwara, et al.; Int. J. Oncol. 48, 854 (2016)