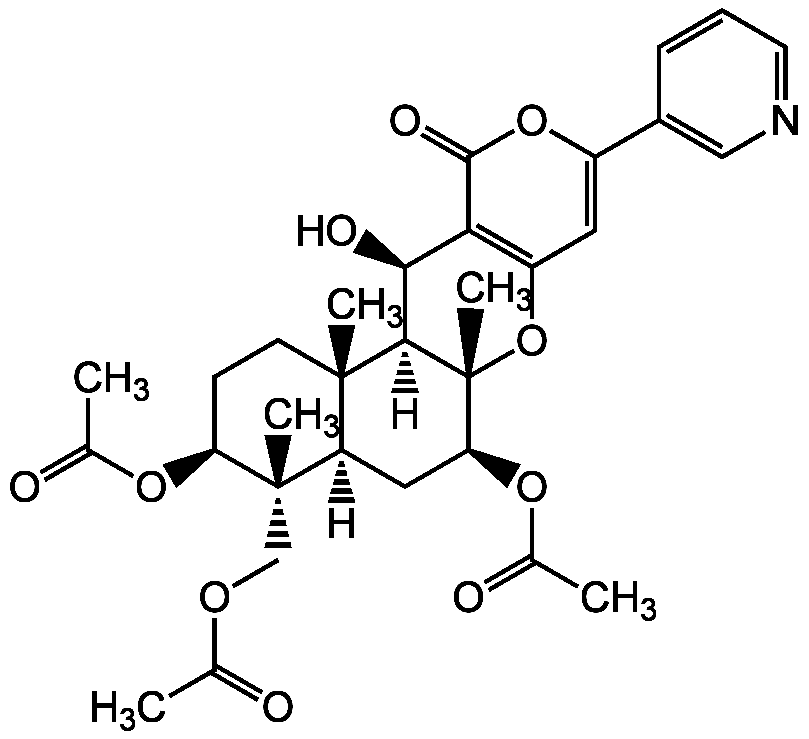
Pyripyropene A
Product Code: AG-CN2-0106
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0106-C250 | 250 ug | £130.00 |
Quantity:
| AG-CN2-0106-M001 | 1 mg | £370.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(+)-Pyripyropene A; PPPA; FO 1289A
Appearance:
Pale pink to rusty brown solid.
CAS:
147444-03-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302
InChi:
InChI=1S/C31H37NO10/c1-16(33)38-15-30(5)22-13-24(40-18(3)35)31(6)27(29(22,4)10-9-23(30)39-17(2)34)26(36)25-21(42-31)12-20(41-28(25)37)19-8-7-11-32-14-19/h7-8,11-12,14,22-24,26-27,36H,9-10,13,15H2,1-6H3/t22-,23+,24+,26+,27-,29+,30+,31-/m1/s1
InChiKey:
PMMQOFWSZRQWEV-RVTXXDJVSA-N
Long Description:
Chemical. CAS: 147444-03-9. Formula: C31H37NO10. MW: 583.6. Isolated from Aspergillus fumigatus FO-1289. Antibiotic. Highly specific inhibitor of acyl-coenzyme A:cholesterol acetyltransferase 2 (ACAT2). Anti-angiogenic. Antiatherogenic and antiatherosclerotic agent. Shows cholesterol-lowering and atheroprotective activities.
MDL:
MFCD00929109
Molecular Formula:
C31H37NO10
Molecular Weight:
583.6
Package Type:
Vial
Precautions:
P264, P301, P312, P330
Product Description:
Antibiotic [1]. Highly specific inhibitor of acyl-coenzyme A:cholesterol acetyltransferase 2 (ACAT2) [1-6, 8, 9]. Anti-angiogenic [10]. Antiatherogenic and antiatherosclerotic agent. Shows cholesterol-lowering and atheroprotective activities. [7, 11, 12].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
[H][C@@]12C[C@H](OC(C)=O)[C@@]3(C)OC4=C([C@H](O)[C@]3([H])[C@@]1(C)CC[C@H](OC(C)=O)C2(C)COC(C)=O)C(=O)OC(=C4)C1=CN=CC=C1
Solubility Chemicals:
Soluble in methanol, chloroform, ethyl acetate or DMSO. Insoluble in water or hexane.
Source / Host:
Isolated from Aspergillus fumigatus FO-1289.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Pyripyropenes, highly potent inhibitors of acyl-CoA:cholesterol acyltransferase produced by Aspergillus fumigatus: S. Omura, et al.; J. Antibiot. (Tokyo) 46, 1168 (1993) | Pyripyropenes, novel inhibitors of acyl-CoA:cholesterol acyltransferase produced by Aspergillus fumigatus. II. Structure elucidation of pyripyropenes A, B, C and D: Y.K. Kim, et al.; J. Antibiot. (Tokyo) 47, 154 (1994) | Pyripyropenes, novel inhibitors of acyl-CoA:cholesterol acyltransferase produced by Aspergillus fumigatus. I. Production, isolation, and biological properties: H. Tomoda, et al.; J. Antibiot. (Tokyo) 47, 148 (1994) | Mass-production of human ACAT-1 and ACAT-2 to screen isoform-specific inhibitor: a different substrate specificity and inhibitory regulation: K.H. Cho, et al.; BBRC 309, 864 (2003) | Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness: A.T. Lada, et al.; J. Lipid Res. 45, 378 (2004) | Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes: T. Ohshiro, et al.; J. Antibiot. (Tokyo) 60, 43 (2007) | Potential therapeutics for obesity and atherosclerosis: inhibitors of neutral lipid metabolism from microorganisms: H. Tomoda & S. Omura; Pharmacol. Ther. 115, 375 (2007) (Review) | Identification of the interaction site within acyl-CoA:cholesterol acyltransferase 2 for the isoform-specific inhibitor pyripyropene A: A. Das, et al.; J. Biol. Chem. 283, 10453 (2008) | Selectivity of pyripyropene derivatives in inhibition toward acyl-CoA:cholesterol acyltransferase 2 isozyme: T. Ohshiro, et al.; J. Antibiot. (Tokyo). 61, 503 (2008) | Pyripyropenes, fungal sesquiterpenes conjugated with alpha-pyrone and pyridine moieties, exhibits anti-angiogenic activity against human umbilical vein endothelial cells: A. Hayashi, et al.; Biol. Pharm. Bull. 32, 1261 (2009) | Pyripyropene A, an acyl-coenzyme A:cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia: T. Ohshiro, et al.; Arterioscler. Thromb. Vasc. Biol. 31, 1108 (2011) | Isoform-specific inhibitors of ACATs: recent advances and promising developments: T. Ohshiro & H. Tomoda; Future Med. Chem. 3, 2039 (2011) (Review)