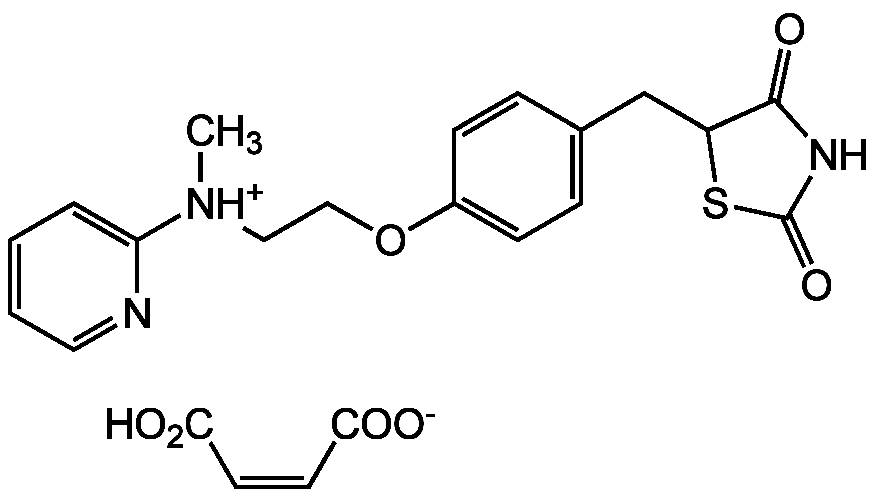
Rosiglitazone . maleate
| Code | Size | Price |
|---|
| AG-CR1-3571-M025 | 25 mg | £60.00 |
Quantity:
| AG-CR1-3571-M100 | 100 mg | £100.00 |
Quantity:
| AG-CR1-3571-G001 | 1 g | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
BRL 49653C; Rosiglitazone XR; Avandia
Appearance:
White to off-white powder.
CAS:
155141-29-0
EClass:
32160000
Form (Short):
liquid
InChi:
InChI=1S/C18H19N3O3S.C4H4O4/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15;5-3(6)1-2-4(7)8/h2-9,15H,10-12H2,1H3,(H,20,22,23);1-2H,(H,5,6)(H,7,8)/b;2-1-
InChiKey:
SUFUKZSWUHZXAV-BTJKTKAUSA-N
Long Description:
Chemical. CAS: 155141-29-0. Formula: C18H19N3O3S . C4H4O4. MW: 357.4 . 116.1. Same activities as rosaglitazone (Prod. No. AG-CR1-3570) but different formulation. Antidiabetic, hypoglycemic agent. Potent and selective peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist. Potent insulin sensitizing agent binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. Ameliorates insulin resistance. Improves blood pressure and vascular function. Enhances proliferation of endogenous neural progenitor cells (NPCs). Anti-inflammatory compound. Has controversial therapeutic effects on the cardiovascular system. Promotes adipocyte differentiation of mesenchymal stem cells (MSCs).
MDL:
MFCD03427306
Molecular Formula:
C18H19N3O3S . C4H4O4
Molecular Weight:
357.4 . 116.1
Package Type:
Vial
Product Description:
Same activities as rosaglitazone (Prod. No. AG-CR1-3570) but different formulation. Antidiabetic, hypoglycemic agent [1, 5]. Potent and selective peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist [2, 3]. Potent insulin sensitizing agent binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. Ameliorates insulin resistance [4-6, 8]. Improves blood pressure and vascular function [7]. Enhances proliferation of endogenous neural progenitor cells (NPCs) [9]. Anti-inflammatory compound [10, 11]. Has controversial therapeutic effects on the cardiovascular system [12]. Promotes adipocyte differentiation of mesenchymal stem cells (MSCs) [13].
Purity:
>98% (NMR)
SMILES:
OC(=O)C=C/C([O-])=O.C[NH+](CCOC1=CC=C(CC2SC(=O)NC2=O)C=C1)C1=CC=CC=N1
Solubility Chemicals:
Soluble in ethanol or buffered aqueous solutions with pH 2.3.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C. Stock solutions are stable for at least 3 months when stored at -20°C.
References
[[omega-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents: B.C. Cantello, et al.; J. Med. Chem. 37, 3977 (1994) | An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPARgamma): J.M. Lehmann, et al.; J. Biol. Chem. 270, 12953 (1995) | Rosiglitazone: J.A. Balfour & G.L. Plosker; Drugs 57, 921 (1999) (Review) | Differential effects of insulin-sensitizers troglitazone and rosiglitazone on ion currents in rat vascular myocytes: G.A. Knock, et al.; Eur. J. Pharmacol. 368, 103 (1999) | Rosiglitazone: an agent from the thiazolidinedione class for the treatment of type 2 diabetes: A. Cheng-Lai & A. Levine; Heart Dis. 2, 326 (2000) (Review) | Rosiglitazone in the treatment of type 2 diabetes mellitus: a critical review: J.M. Malinowski & S. Bolesta; Clin. Ther. 22, 1151 (2000) (Review) | PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice: M.J. Ryan, et al.; Hypertension 3, 661 (2004) | Rosiglitazone: a review of its use in type 2 diabetes mellitus: E.D. Deeks & S.J. Keam; Drugs 67, 2747 (2007) (Review) | Rosiglitazone enhances the proliferation of neural progenitor cells and inhibits inflammation response after spinal cord injury: Q.Q. Meng, et al.; Neurosci. Lett. 503, 191 (2011) | Inhibitory effects of rosiglitazone on lipopolysaccharide-induced inflammation in a murine model and HK-2 cells: W.M. Wang, et al.; Am. J. Nephrol. 34, 152 (2011) | PPARgamma agonist rosiglitazone ameliorates LPS-induced inflammation in vascular smooth muscle cells via the TLR4/TRIF/IRF3/IP-10 signaling pathway: Y. Ji, et al.; Cytokine 55, 409 (2011) | PPARgamma activator, rosiglitazone: Is it beneficial or harmful to the cardiovascular system? S. Palee, et al.; World J. Cardiol. 3, 144 (2011) | Rosiglitazone-induced adipogenesis in a bone marrow mesenchymal stem cell line: D. Wang, et al.; Biomed. Sci. Instrum. 47, 213 (2011) | Rosiglitazone requires hepatocyte PPARgamma expression to promote steatosis in male mice with diet-induced obesity: S.M. Lee, et al.; Endocrinology, 175, (2021)
Related Products
| Product Name | Product Code | Supplier | Ciglitazone | AG-CR1-0033 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pioglitazone | AG-CR1-0067 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rosiglitazone | AG-CR1-3570 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||