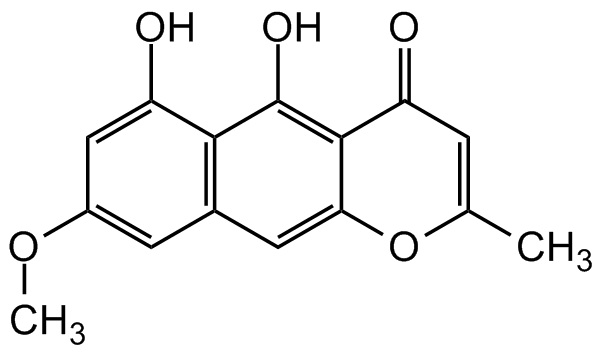
Rubrofusarin
| Code | Size | Price |
|---|
| BVT-0395-M001 | 1 mg | £130.00 |
Quantity:
| BVT-0395-M005 | 5 mg | £365.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 258316
Appearance:
Orange needles.
CAS:
3567-00-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C15H12O5/c1-7-3-10(16)14-12(20-7)5-8-4-9(19-2)6-11(17)13(8)15(14)18/h3-6,17-18H,1-2H3
InChiKey:
FPNKCZKRICBAKG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 3567-00-8. Formula: C15H12O5. MW: 272.3. Isolated from Fusarium graminearum. Monomer of aurofusarin. Mycotoxin. Anticancer and antimycobacterial compound in vivo. Moderate tyrosinase inhibitor. Anti-estrogenic.
MDL:
MFCD30146414
Molecular Formula:
C15H12O5
Molecular Weight:
272.3
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Monomer of aurofusarin. Mycotoxin. Anticancer and antimycobacterial compound in vivo. Moderate tyrosinase inhibitor. Anti-estrogenic.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
COC1=CC2=C(C(O)=C1)C(O)=C1C(=O)C=C(C)OC1=C2
Solubility Chemicals:
Soluble in DMSO. Sparingly soluble in usual organic solvents.
Source / Host:
Isolated from Fusarium graminearum.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Structure of rubrofusarin: G.H. Stout, et al.; Chem. Indust. 1961, 289 (1961). | Metabolic products of fungi. XXV. Synthesis of rubrofusarin and its derivatives: S. Shibata, et al.; Chem. Pharm. Bul.l (Tokyo) 15, 1757 (1967) | The biosynthesis of rubrofusarin, a polyketide naphthopyrone from Fusarium culmorum: F.J. Leeper & J. Staunton; J. Chem. Soc. Perkin 1 1984, 2919 (1984). | Biometic syntheses of the polyketide fungal metabolites alternariol and rubrofusarin: C. Abell, et al.; J. Chem. Soc. Chem. Comm. 1986, 15 (1986) | Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant St. aureus: T. Hatano, et al.; Chem. Pharm. Bull. 47, 1121 (1999) | A new naphthopyrone derivative from Cassia quinquangulata and structural revision of quinquangulin and its glycosides: X.C. Li, et al.; J. Nat. Prod. 64, 1153 (2001) | Antimycobacterial naphthopyrones from Senna oblique: J.G. Graham, et al.; J. Nat. Prod. 67, 225 (2004) | Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents: A.M. El-Halawany, et al.; Chem. Pharm. Bull. (Tokyo) 55, 1476 (2007) | In vitro cytotoxicity of fungal spoiling maize silage: R.R. Rasmussen, et al.; Food Chem. Toxicol. 49, 31 (2010) | Three dimeric naphtho-gamma-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata: H.-B. Huang, et al.; Planta Med 76, 1888 (2010) | Two novel classes of enzymes are required for the biosynthesis of aurofusarin in Fusarium graminearum: R.J.N. Frandsen, et al.; J. Biol. Chem. 286, 10419 (2011)
Related Products
| Product Name | Product Code | Supplier | Helvolic acid | BVT-0435 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|