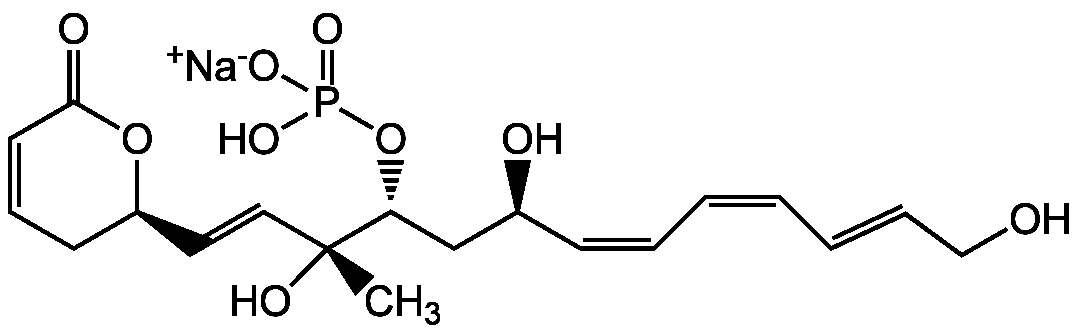
Fostriecin
Product Code: AG-CN2-0057
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0057-C010 | 10 ug | £170.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Phosphotrienin; CI-920; CL-1565A; NSC-339638; PD 110,161; Pyranone phosphate
Appearance:
Colorless solid.
CAS:
87860-39-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Hygroscopic.Keep cool and dry.
Hazards:
H302
InChi:
InChI=1/C19H27O9P.Na/c1-19(23,12-11-16-9-7-10-18(22)27-16)17(28-29(24,25)26)14-15(21)8-5-3-2-4-6-13-20;/h2-8,10-12,15-17,20-21,23H,9,13-14H2,1H3,(H2,24,25,26);/q;+1/p-1/b3-2-,6-4+,8-5-,12-11+;/t15-,16+,17+,19+;/m0./s1
InChiKey:
XBUIKNRVGYFSHL-CZYWAGOEBB
Long Description:
Chemical. CAS: 87860-39-7. Formula: C19H26O9PNa. MW: 452.4. Isolated from Streptomyces pulveraceous subsp. fostreus. Antibiotic. Anticancer compound. Antifungal. Catalytic inhibitor of topoisomerase II (IC50 = 40 µM). Potent protein phosphatase 2A (PP2A) (IC50 = 1.5 nM) and 4 (PP4) (IC50 = 3 nM) inhibitor. Weak protein phosphatase type 1 (PP1) inhibitor (IC50=131µM). No apparent inhibition on PP2B. Mitotic entry checkpoint inhibitor. Mediates cell cycle arrest at G2-M-phase. Ischemia protective. The PP2A binding site is different from that of okadaic acid
MDL:
MFCD01939908
Molecular Formula:
C19H26O9PNa
Molecular Weight:
452.4
Other data:
Use only fresh solutions.
Package Type:
Vial
Precautions:
P264, P301, P312, P330
Product Description:
Antibiotic [1, 2, 18]. Anticancer compound [1-5, 8, 9, 11]. Antifungal [6]. Catalytic inhibitor of topoisomerase II (IC50 = 40 µM) [7, 16]. Potent protein phosphatase 2A (PP2A) (IC50 = 1.5 nM) and 4 (PP4) (IC50 = 3 nM) inhibitor [10, 12, 15]. Weak protein phosphatase type 1 (PP1) inhibitor (IC50=131µM). No apparent inhibition on PP2B [10, 12]. Mitotic entry checkpoint inhibitor [10]. Mediates cell cycle arrest at G2-M-phase [14]. Ischemia protective [13]. The PP2A binding site is different from that of okadaic acid [17, 19]
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
[Na+].C[C@@](O)(C=C[C@H]1CC=CC(=O)O1)[C@@H](C[C@@H](O)C=C/C=CC=CCO)OP(O)([O-])=O
Solubility Chemicals:
Soluble in water, methanol or ethanol.
Source / Host:
Isolated from Streptomyces pulveraceous.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Novel antitumor agents CI-920, PD 113,270 and PD 113,271. I. Taxonomy, fermentation and biological properties: J.B. Tunac, et al.; J. Antibiot. (Tokyo) 36, 1595 (1983) | Novel antitumor agents CI-920, PD 113,270 and PD 113,271. II. Isolation and characterization: S.S. Stampwala, et al.; J. Antibiot. (Tokyo) 36, 1601 (1983) | Studies on the biochemical mechanism of the novel antitumor agent, CI- 920: D.W. Fry, et al.; Cancer Chemother. Pharmacol. 13, 171 (1984) | Anticancer activity of the structurally novel antibiotic Cl-920 and its analogues: W.R. Leopold, et al.; Cancer Res. 44, 1928 (1984) | In vitro activity of the novel antitumor antibiotic fostriecin (CI-920) in a human tumor cloning assay: W. Scheithauer, et al.; Eur. J. Cancer Clin. Oncol. 22, 921 (1986) | Antimycotic effects of the novel antitumor agents fostriecin (CI-920), PD 113,270 and PD 113,271: S.W. Mamber, et al.; J. Antibiot. (Tokyo) 39, 1467 (1986) | Inhibition of type II topoisomerase by fostriecin: T.J. Boritzki, et al.; Biochem. Pharmacol. 37, 4063 (1988) | Cytostatic and cytotoxic effects of fostriecin on human promyelocytic HL-60 and lymphocytic MOLT-4 leukemic cells: M.A. Hotz, et al.; Cancer Res. 52, 1530 (1992) | Changes in nuclear chromatin related to apoptosis or necrosis induced by the DNA topoisomerase II inhibitor fostriecin in MOLT-4 and HL-60 cells are revealed by altered DNA sensitivity to denaturation: M.A. Hotz, et al.; Exp. Cell Res. 201, 184 (1992) | Antitumor drug fostriecin inhibits the mitotic entry checkpoint and protein phosphatases 1 and 2A: M. Roberge, et al.; Cancer Res. 54, 6115 (1994) | Fostriecin: a review of the preclinical data: R.S. de Jong, et al.; Anticancer Drugs 8, 413 (1997) | Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A: A.H. Walsh, et al.; FEBS Lett. 416, 230 (1997) | Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia: C. Weinbrenner, et al.; Circulation 98, 899 (1998) | Fostriecin-mediated G2-M-phase growth arrest correlates with abnormal centrosome replication, the formation of aberrant mitotic spindles, and the inhibition of serine/threonine protein phosphatase activity: A. Cheng, et al.; Cancer Res. 58, 3611 (1998) | Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters: C.J. Hastie & P.T. Cohen; FEBS Lett. 431, 357 (1998) | Phase I and pharmacokinetic study of the topoisomerase II catalytic inhibitor fostriecin: R.S. de Jong, et al.; Br. J. Cancer 79, 882 (1999) | The predicted beta12-beta13 loop is important for inhibition of PP2Acalpha by the antitumor drug fostriecin: D.R. Evans & J.A. Simon; FEBS Lett. 498, 110 (2001) | Fostriecin: chemistry and biology: D.S. Lewy, et al.; Curr. Med. Chem. 9, 2005 (2002) | Antitumor antibiotic fostriecin covalently binds to cysteine-269 residue of protein phosphatase 2A catalytic subunit in mammalian cells: T. Takeuchi, et al.; Bioorg. Med. Chem. 17, 8113 (2009)