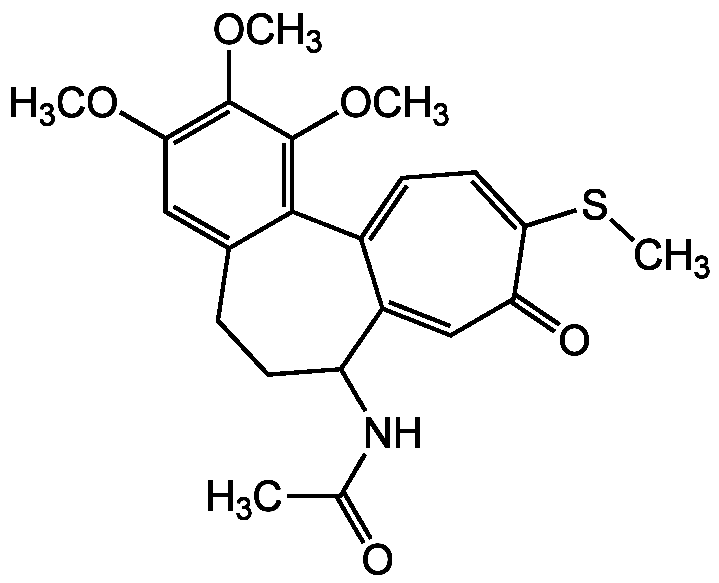
Thiocolchicine
Product Code: AG-CN2-0074
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0074-M005 | 5 mg | £40.00 |
Quantity:
| AG-CN2-0074-M025 | 25 mg | £135.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
10-Demethoxy-10-methylthiocolchicine; NSC 186301
Appearance:
Yellow solid.
CAS:
2730-71-4
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H300, H318, H330
InChi:
InChI=1S/C22H25NO5S/c1-12(24)23-16-8-6-13-10-18(26-2)21(27-3)22(28-4)20(13)14-7-9-19(29-5)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)
InChiKey:
CMEGANPVAXDBPL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 2730-71-4. Formula: C22H25NO5S. MW: 415.5. Semisynthetic. Antimitotic alkaloid. Tubulin polymerization and microtubule assembly inhibitor. Axonal cytoskeleton modulator. Inhibitor of autophagosome-lysosome fusion. Apoptosis inducer. Thiocolchicine-dimers were shown to be potent topoisomerase I inhibitors.
MDL:
MFCD01736965
Molecular Formula:
C22H25NO5S
Molecular Weight:
415.5
Package Type:
Vial
PG:
III
Precautions:
P260, P301, P310, P304, P340
Product Description:
Antimitotic alkaloid. Tubulin polymerization and microtubule assembly inhibitor. Axonal cytoskeleton modulator. Inhibitor of autophagosome-lysosome fusion. Apoptosis inducer. Thiocolchicine-dimers were shown to be potent topoisomerase I inhibitors.
Purity:
>95% (NMR)
Signal word:
Danger
SMILES:
COC1=C(OC)C(OC)=C2C(CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C23)=C1
Solubility Chemicals:
Soluble in ethanol or acetone. Almost insoluble in water or ether.
Source / Host:
Semisynthetic.
Transportation:
Excepted Quantity
UN Nummer:
UN 1544
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Association of thiocolchicine with tubulin: R.M. Chabin & S.B. Hastie; BBRC 161, 544 (1989) | Effect of tubulin binding and self-association on the near-ultraviolet circular dichroic spectra of colchicine and analogues; R.M. Chabin, et al.; Biochemistry 29, 1869 (1990) | N-acetylcolchinol O-methyl ether and thiocolchicine, potent analogs of colchicine modified in the C ring. Evaluation of the mechanistic basis for their enhanced biological properties: G.J. Kang, et al.; J. Biol. Chem. 265, 10255 (1990) | Antitumor agents-CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis: Q. Shi, et al.; Bioorg. Med. Chem. 5, 2277 (1997) | Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines: R. De Vincenzo, et al.; Anticancer Drug Des. 13, 19 (1998) | Biological evaluation on different human cancer cell lines of novel colchicine analogs: R. De Vincenzo, et al.; Oncol. Res. 11, 145 (1999) | Effects of thiocolchicine on axonal cytoskeleton of the rat peroneus nerve: P. Ferri, et al.; Exp. Toxicol. Pathol. 54, 211 (2002) | Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors: G. Raspaglio, et al.; Biochem. Pharmacol. 69, 113 (2005) | Inhibitors of tubulin polymerization: synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III: D. Passarella, et al.; Bioorg. Med. Chem. 16, 6269 (2008) | Synthesis and biological evaluation of novel thiocolchicine-podophyllotoxin conjugates: D. Passarella, et al.; Eur. J. Med. Chem. 45, 219 (2010)
Related Products
| Product Name | Product Code | Supplier | Colchicine | AG-CN2-0048 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiocolchicoside | AG-CN2-0076 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Colcemid | AG-CR1-3567 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||