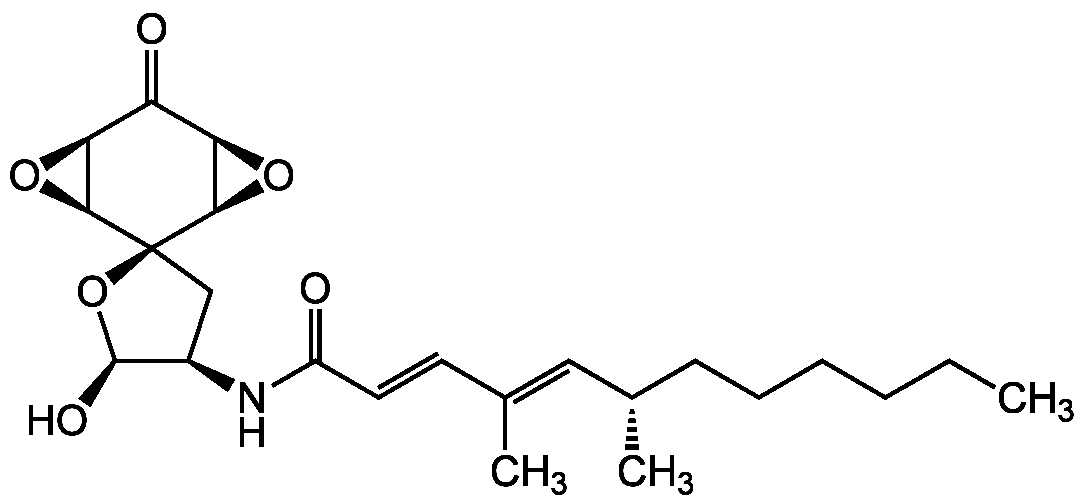
Aranorosin
Product Code: AG-CN2-0114
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0114-C250 | 250 ug | £60.00 |
Quantity:
| AG-CN2-0114-M001 | 1 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
White solid.
CAS:
117184-53-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C23H33NO6/c1-4-5-6-7-8-13(2)11-14(3)9-10-16(25)24-15-12-23(30-22(15)27)20-18(28-20)17(26)19-21(23)29-19/h9-11,13,15,18-22,27H,4-8,12H2,1-3H3,(H,24,25)/b10-9+,14-11+/t13-,15?,18-,19+,20-,21+,22?,23?/m1/s1
InChiKey:
JHTWWPWUODMKEO-PHJKOLFRSA-N
Long Description:
Chemical. CAS: 117184-53-9. Formula: C23H33NO6. MW: 419.5. Isolated from Gymnascella sp. Antibiotic. Antibacterial. Antifungal. Anticancer compound. Inhibits anti-apoptotic functions regulated by Bcl-2. Circumvents arbekacin (ABK)-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6')/APH(2'').
MDL:
MFCD01746201
Molecular Formula:
C23H33NO6
Molecular Weight:
419.5
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Antibiotic [1, 2]. Antibacterial [1-6]. Antifungal [1-6]. Anticancer compound [3, 4]. Inhibits anti-apoptotic functions regulated by Bcl-2 [7]. Circumvents arbekacin (ABK)-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6')/APH(2'') [8].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
CCCCCC[C@H](C)C=C(/C)C=CC(=O)N[C@@H]1C[C@]2(O[C@@H]1O)[C@H]1O[C@H]1C(=O)[C@H]1O[C@@H]21
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Gymnascella sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 3 years after receipt when stored at -20°C.
References
Aranorosin, a novel antibiotic from Pseudoarachniotus roseus. I. Taxonomy, fermentation, isolation, chemical and biological properties: K. Roy, et al.; J. Antibiot. (Tokyo) 41, 1780 (1988) | Aranorosin, a novel antibiotic from Pseudoarachniotus roseus. II. Structure elucidation: H.W. Fehlhaber, et al.; J. Antibiot. (Tokyo) 41, 1785 (1988) | Structure of aranorosin, a new antibiotic of a novel skeletal type: H.W. Fehlhaber, et al.; JACS 110, 8242 (1988) | Stereoselective synthesis of the functionalized spirocyclic core of aranorosin: P. Wipf & Y. Kim; J. Org. Chem. 58, 1649 (1993) | Structure of a model for the aranorosin nucleus: R. C. Haltiwanger, et al.; Acta Cryst. C 50, 274 (1994) | Structure-activity relationships of new aranorosin analogs to antifungal activity: E.K. Vijayakumar, et al.; J. Antibiot. (Tokyo) 51, 522 (1998) | Aranorosin and a novel derivative inhibit the anti-apoptotic functions regulated by Bcl-2: T. Nakashima, et al.; BBRC 377, 1085 (2008) | Aranorosin circumvents arbekacin-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6')/APH(2''): T. Suga, et al.; J. Antibiot. (Tokyo) 65, 527 (2012)