Salsalate
| Code | Size | Price |
|---|
| AG-CR1-3574-G001 | 1 g | £35.00 |
Quantity:
| AG-CR1-3574-G005 | 5 g | £75.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Salicylsalicylic acid; NSC 49171; BRN 2590908; 2-Hydroxy-2-carboxyphenyl ester-benzoic acid
Appearance:
White to off-white powder.
CAS:
552-94-3
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS09
Handling Advice:
Keep cool and dry.
Hazards:
H302, H400
InChi:
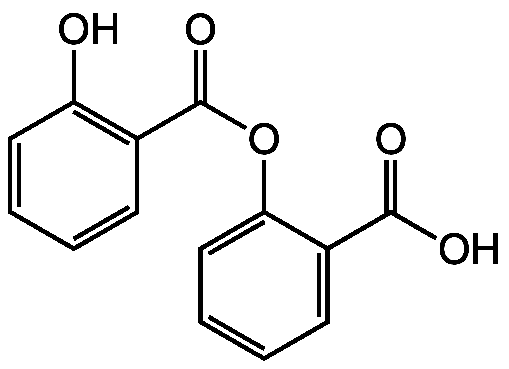
InChI=1/C14H10O5/c15-11-7-3-1-5-9(11)14(18)19-12-8-4-2-6-10(12)13(16)17/h1-8,15H,(H,16,17)
InChiKey:
WVYADZUPLLSGPU-UHFFFAOYAY
Long Description:
Chemical. CAS: 552-94-3. Formula: C14H10O5. MW: 258.2. Nonacetylated salicylate. Non-steroidal anti-inflammatory drug (NSAID). Reduces pain and inflammation caused by conditions such as rheumatoid arthritis, osteoarthritis and related rheumatic conditions. Prostaglandin synthesis inhibitor in vivo. Inactivates cyclooxygenase-1 (COX-1) and -2 (COX-2). IKKbeta/NF-kappaB inhibitor (at significantly higher concentrations than required for COX inhibition). Used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes. Reduces blood glucose, triglyceride, free fatty acid and C-reactive protein concentrations, improves glucose utilization and increases circulating insulin and adiponectin concentrations in obese adults at risk for the development of type 2 diabetes as well as for patients with type 2 diabetes. Dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact.
MDL:
MFCD00020252
Molecular Formula:
C14H10O5
Molecular Weight:
258.2
Package Type:
Vial
Precautions:
P264, P273, P301, P312, P330
Product Description:
Nonacetylated salicylate. Non-steroidal anti-inflammatory drug (NSAID). Reduces pain and inflammation caused by conditions such as rheumatoid arthritis, osteoarthritis and related rheumatic conditions. Prostaglandin synthesis inhibitor in vivo. Inactivates cyclooxygenase-1 (COX-1) and -2 (COX-2). IKKbeta/NF-kappaB inhibitor (at significantly higher concentrations than required for COX inhibition). Used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes. Reduces blood glucose, triglyceride, free fatty acid and C-reactive protein concentrations, improves glucose utilization and increases circulating insulin and adiponectin concentrations in obese adults at risk for the development of type 2 diabetes as well as for patients with type 2 diabetes. Dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact.
Purity:
>98%
Signal word:
Warning
SMILES:
OC(=O)C1=CC=CC=C1OC(=O)C1=C(O)C=CC=C1
Solubility Chemicals:
Soluble in DMSO, ethanol or dimethylformamide. Sparingly soluble in water.
Transportation:
Non-hazardous
UN Nummer:
UN 3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study: S. Roth, et al.; Semin. Arth. Rheum. 19, 11 (1990) | Inhibition of NF-kappa B by sodium salicylate and aspirin: E. Kopp & S. Ghosh; Science 265, 956 (1994) | Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration: J.W. Pierce, et al.; J. Immunol. 156, 3961 (1996) | The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta: M.J. Yin, et al.; Nature 396, 77 (1998) | Salsalate improves glycemia and inflammatory parameters in obese young adults: A. Fleischman, et al.; Diabetes Care 31, 289 (2008) | Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes: A.B. Goldfine, et al.; Clin. Transl. Sci. 1, 36 (2008) | The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study: J. Koska, et al.; Diabetologia 52, 385 (2009) | Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans: G.L. Pierce, et al.; Circulation 119, 1284 (2009) | The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial: A.B. Goldfine, et al.; Ann. Intern. Med. 152, 346 (2010) | Potential role of salicylates in type 2 diabetes: M.M. Rumore & K.S. Kim; Ann. Pharmacother. 44, 1207 (2010) (Review) | Effects of salsalate therapy on recovery from vascular injury in female Zucker fatty rats: S.N. Murthy, et al.; Diabetes 59, 3240 (2010) | Stimulation of human whole-body energy expenditure by salsalate is fueled by higher lipid oxidation under fasting conditions and by higher oxidative glucose disposal under insulin-stimulated conditions: R.C. Meex, et al.; J. Clin. Endocrinol. Metab. 96, 1415 (2011) | Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans: W. Chai, et al.; Diabetes Care 34, 1634 (2011) | Modeling diabetes disease progression and salsalate intervention in Goto-Kakizaki rats: Y. Cao, et al.; J. Pharmacol. Exp. Ther. 339, 896 (2011) | Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes: E. Faghihimani, et al.; Acta Diabetol. 50, 537 (2013) | Salicylate downregulates 11beta-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization: M. Nixon, et al.; Diabetes 61, 790 (2012) | The ancient drug salicylate directly activates AMP-activated protein kinase: S.A. Hawley, et al.; Science 336, 918 (2012) | Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes: E. Faghihimani, et al.; Endocr. Pract. 18, 826 (2012) | Reducing acetylated tau is neuroprotective in brain injury: MK. Shin, et al.; Cell 184, 2715 (2021)
Related Products
| Product Name | Product Code | Supplier | Empagliflozin | AG-CR1-3619 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|