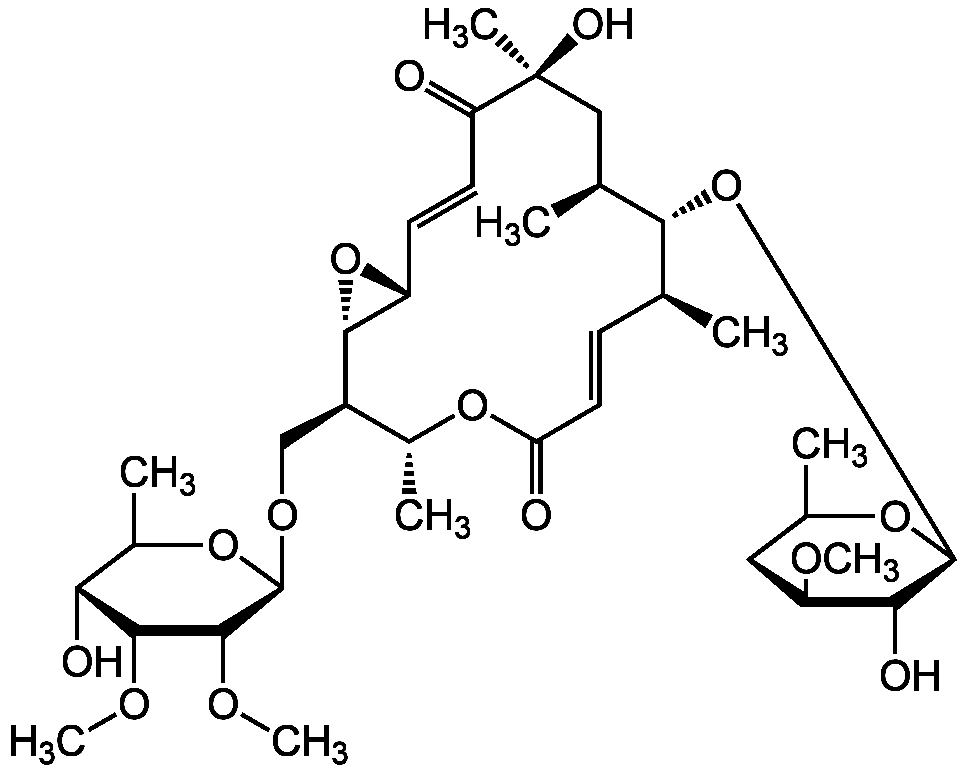
Chalcomycin
| Code | Size | Price |
|---|
| BVT-0263-C500 | 500 ug | £130.00 |
Quantity:
| BVT-0263-M001 | 1 mg | £205.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Aldgamycin D; NSC 150439
Appearance:
Beige solid.
CAS:
20283-48-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H319, H335
InChi:
InChI=1S/C35H56O14/c1-17-10-13-26(37)46-20(4)22(16-44-34-32(43-9)31(42-8)27(38)21(5)47-34)30-23(48-30)11-12-25(36)35(6,40)15-18(2)29(17)49-33-28(39)24(41-7)14-19(3)45-33/h10-13,17-24,27-34,38-40H,14-16H2,1-9H3/b12-11+,13-10+/t17-,18-,19+,20+,21+,22+,23+,24?,27+,28+,29+,30-,31+,32+,33-,34+,35-/m0/s1
InChiKey:
KLGADJPDTCIJLO-GQVCZQSUSA-N
Long Description:
Chemical. CAS: 20283-48-1. Formula: C35H56O14. MW: 700.8. Isolated from Streptomyces bikiniensis. 16-membered macrolide antibiotic. Broad spectrum antimicrobial. Antibacterial and antifungal. Protein biosynthesis inhibitor.
MDL:
MFCD00038602
Molecular Formula:
C35H56O14
Molecular Weight:
700.8
Package Type:
Plastic Vial
Precautions:
P261, P271, P280, P312
Product Description:
16-membered macrolide antibiotic. Broad spectrum antimicrobial. Antibacterial and antifungal. Protein biosynthesis inhibitor.
Purity:
>96% (HPLC)
Signal word:
Warning
SMILES:
CO[C@@H]1CC(C)O[C@@H](O[C@H]2[C@@H](C)C[C@](C)(O)C(=O)C=C[C@H]3O[C@H]3[C@H](CO[C@@H]3OC(C)[C@@H](O)[C@H](OC)C3OC)[C@@H](C)OC(=O)C=C[C@@H]2C)C1O
Solubility Chemicals:
Soluble in DMSO or acetone.
Source / Host:
Isolated from Streptomyces bikiniensis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
The structure of chalcomycin: P.W.K. Woo, et al.; JACS 86, 2726 (1964) | Cross resistance pattern towards anticancer drugs of a human carcinoma multidrug-resistant cell line: R.S. Gupta, et al.; Br. J. Cancer 58, 441 (1988) | Chalcomycin: single-crystal x-ray crystallographic analysis; biosynthetic and stereochemical correlations with other polyoxo macrolide antibiotics: P.W.K. Woo & J.R. Rubin; Tetrahedron 52, 3857 (1996) | Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064: R.N. Asolkar, et al.; J. Antibiot. (Tokyo) 55, 893 (2002) | Chalcomycin biosynthesis gene cluster from Streptomyces bikiniensis: Novel features of an unusual ketolide produced through expression of the chm polyketide synthase in streptomyces fradiae: S.L. Ward, et al.; Antimicrob. Agents Chemoth. 48, 4703 (2004) | Aldgamycin I, an antibacterial 16-membered macrolide from the abandoned mine bacterium, Streptomyces sp. KMA-001: J.-S. Park, et al.; J. Antibiot. (Tokyo) 62, 171 (2009) | Imaging secondary metabolism of Streptomyces sp. Mg1 during cellular lysis and colony degradation of competing Bacillus subtilis: S.R. Barger, et al.; Antonie Van Leeuwenhoek 102, 435 (2012)