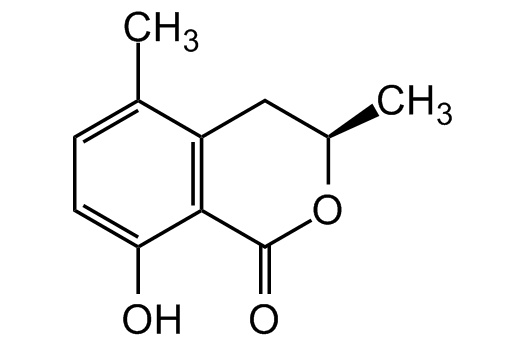
5-Methylmellein
| Code | Size | Price |
|---|
| BVT-0413-M001 | 1 mg | £85.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
5- Methylochracin; 3,4-Dihydro-8-hydroxy-3,5-dimethyl-1H-2-benzopyran1- one; 3,4-Dihydro-8-hydroxy-3,5-dimethylisocoumarin
Appearance:
Pale yellow powder.
CAS:
7734-92-1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C11H12O3/c1-6-3-4-9(12)10-8(6)5-7(2)14-11(10)13/h3-4,7,12H,5H2,1-2H3/t7-/m1/s1
InChiKey:
YETSBBYQOFXYGV-SSDOTTSWSA-N
Long Description:
Chemical. CAS: 7734-92-1. Formula: C11H12O3. MW: 192.2. Isolated from Fusicoccum amygdalis. Widespread fungal pentaketide. Weak antibacterial, antifungal and antiviral activity. Specific inhibitor of human DNA polymerase lambda. Weak antigerminative activity.
MDL:
MFCD24849959
Molecular Formula:
C11H12O3
Molecular Weight:
192.2
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Widespread fungal pentaketide. Weak antibacterial, antifungal and antiviral activity. Specific inhibitor of human DNA polymerase lambda. Weak antigerminative activity.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
C[C@@H]1CC2=C(C(O)=CC=C2C)C(=O)O1
Solubility Chemicals:
Soluble in DMSO, chloroform or dichloromethane.
Source / Host:
Isolated from Fusicoccum amygdalis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
5-Methylmellein, a new natural dihydroisocoumarin: A. Ballio, et al.; Tetrahedron Lett. 1966, 3723 (1966) | (-)-5-Methylmellein and catechol derivatives from four semecarpus species: R.C. Carpenter, et al.; Phytochemistry 19, 445 (1980) | 3-Methyl-3,4-dihydroisocoumarins and related compounds from the ascomycete family xylariaceae: J.R. Anderson & R.L. Edwards; J. Chem. Soc. Perkin Trans I 1983, 2185 (1983) | Dihydroisocoumarins from fungi: isolation, structure elucidation, circular dichroism and biological activity: K. Krohn, et al.; Phytochemistry 45, 313 (1997) | Nodulisporol and nodulisporone, novel specific inhibitors of human DNA polymerase lambda from a fungus, Nodulisporium sp.: S. Kamisuki, et al.; Bioorg. Med. Chem. 15, 3109 (2007) | Bioactive aromatic derivatives from endophytic fungus, Cytospora sp.: S. Lu, et al.; Nat. Prod. Commun. 6, 661 (2011) | Guaiane sesquiterpenes from Biscogniauxia nummularia featuring potent antigerminative activity: S. Amand, et al.; J. Nat. Prod. 75, 798 (2012)