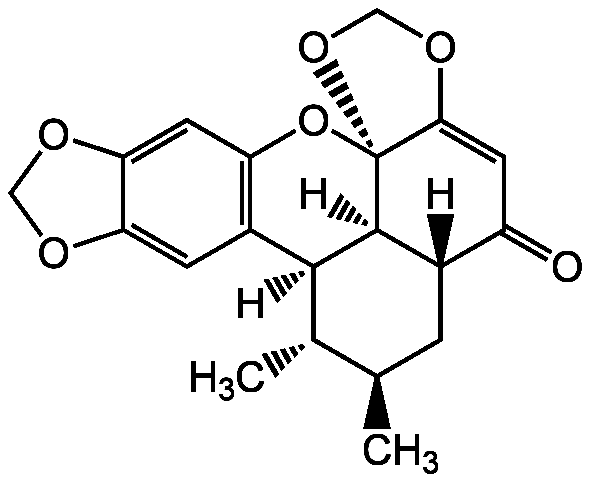
Sauchinone
Product Code: AG-CN2-0032
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0032-M001 | 1 mg | £35.00 |
Quantity:
| AG-CN2-0032-M005 | 5 mg | £85.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1'-Epimer dihydrocarpanone
Appearance:
White to off-white powder.
CAS:
177931-17-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C20H20O6/c1-9-3-11-13(21)5-17-20(25-8-24-17)19(11)18(10(9)2)12-4-15-16(23-7-22-15)6-14(12)26-20/h4-6,9-11,18-19H,3,7-8H2,1-2H3/t9-,10+,11+,18+,19+,20+/m1/s1
InChiKey:
GMTJIWUFFXGFHH-WPAOEJHSSA-N
Long Description:
Chemical. CAS: 177931-17-8. Formula: C20H20O6. MW: 356.4. Isolated from Saururus chinensis. Hepatoprotective antioxidant. Anti-inflammatory. Suppresses NF-kB p65 activity. Neuroprotective. Apoptosis inhibitor. Bone resorption/osteoclastogenesis inhibitor. Reduces ROS formation. Antiseptic. AMP-activated kinase (AMPK) activator. Akt phosphorylation inhibitor.
MDL:
MFCD08702698
Molecular Formula:
C20H20O6
Molecular Weight:
356.4
Package Type:
Vial
Product Description:
Hepatoprotective antioxidant [1, 2, 9]. Anti-inflammatory. Suppresses NF-kB p65 activity [3, 5]. Neuroprotective. Apoptosis inhibitor [4]. Bone resorption/osteoclastogenesis inhibitor. Reduces ROS formation [6]. Antiseptic [7]. AMP-activated kinase (AMPK) activator [8]. Akt phosphorylation inhibitor [10]. Arginase II inhibitor [11].
Purity:
>98% (HPLC)
SMILES:
[H][C@]12C[C@@H](C)[C@H](C)[C@@]3([H])C4=C(O[C@]5(OCOC5=CC1=O)[C@@]23[H])C=C1OCOC1=C4
Solubility Chemicals:
Soluble in DMSO or chloroform.
Source / Host:
Isolated from Saururus chinensis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Sauchinone, a lignan from Saururus chinensis, attenuates CCl4-induced toxicity in primary cultures of rat hepatocytes: S.H. Sung, et al.; Biol. Pharm. Bull. 23, 666 (2000) | Hepatoprotective diastereomeric lignans from Saururus chinensis herbs: S.H. Sung & Y.C. Kim; J. Nat. Prod. 63, 1019 (2000) | Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation: A.K. Lee, et al.; Br. J. Pharmacol. 139, 11 (2003) | Sauchinone, a lignan from Saururus chinensis, inhibits staurosporine-induced apoptosis in C6 rat glioma cells: H. Song, et al.; Biol. Pharm. Bull. 26, 1428 (2003) | Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB: B.Y. Hwang, et al.; Planta Med. 69, 1096 (2003) | Inhibition of osteoclast differentiation and bone resorption by sauchinone: K.Y. Han, et al.; Biochem. Pharmacol. 74, 911 (2007) | Protective effect of lignans against sepsis from the roots of Saururus chinensis: C.S. Seo, et al.; Biol. Pharm. Bull. 31, 523 (2008) | Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury: Y.W. Kim, et al.; Free Radic. Biol. Med. 47, 1082 (2009) | Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCdelta-GSK3beta pathway: H.Y. Kay, et al.; Br. J. Pharmacol. 163, 1653 (2011) | Sauchinone suppresses lipopolysaccharide-induced inflammatory responses through Akt signaling in BV2 cells: E.Y. Yang, et al.; Int. Immunopharmacol. 14, 188 (2012) | Arginase II inhibitory activity of phenolic compounds from Saururus chinensis: C. J. Lim, et al.; Bull. Korean Chem. Soc. 33, 3079 (2012) | Pharmacokinetics and pharmacodynamics of promising Arginase Inhibitors: K.S. Abdelkawy, et al.; Eur. J. Drug Metab. Pharmacokinet. 42, 355 (2017) Review
Related Products
| Product Name | Product Code | Supplier | Swinholide A | AG-CN2-0035 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|