Siamycin I
Product Code: AG-CN2-0146
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0146-C250 | 250 ug | £80.00 |
Quantity:
| AG-CN2-0146-M001 | 1 mg | £220.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
MS-271; FR 901724; BMY 29304
Appearance:
White solid.
CAS:
164802-68-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C97H131N23O26S4/c1-11-50(8)80-96(144)119-79(49(6)7)95(143)117-71(93(141)112-63(32-54-22-16-13-17-23-54)87(135)115-66(97(145)146)34-56-37-99-59-25-19-18-24-58(56)59)46-150-149-45-70-92(140)113-64(35-72(98)123)88(136)114-65-36-73(124)108-69(91(139)110-60(30-47(2)3)83(131)101-41-77(128)118-78(48(4)5)94(142)103-40-75(126)107-67(42-121)90(138)116-70)44-148-147-43-68(109-76(127)38-100-81(129)51(9)104-86(134)62(111-89(65)137)31-53-20-14-12-15-21-53)84(132)102-39-74(125)106-61(33-55-26-28-57(122)29-27-55)85(133)105-52(10)82(130)120-80/h12-29,37,47-52,60-71,78-80,99,121-122H,11,30-36,38-46H2,1-10H3,(H2,98,123)(H,100,129)(H,101,131)(H,102,132)(H,103,142)(H,104,134)(H,105,133)(H,106,125)(H,107,126)(H,108,124)(H,109,127)(H,110,139)(H,111,137)(H,112,141)(H,113,140)(H,114,136)(H,115,135)(H,116,138)(H,117,143)(H,118,128)(H,119,144)(H,120,130)(H,145,146)/t50-,51-,52-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,78-,79-,80-/m0/s1
InChiKey:
TXYRKTDGDMHVHR-NEKRQKPVSA-N
Long Description:
Chemical. CAS: 164802-68-0. Formula: C97H131N23O26S4. MW: 2163.5. Isolated from Streptomyces sp. Antibiotic. Lariat peptide antiviral. Anti-HIV and anti-HSV (Herpes simplex virus) agent. Fsr quorum sensing inhibitor. Calmodulin-activated myosin light chain kinase (MLCK) inhibitor.
Molecular Formula:
C97H131N23O26S4
Molecular Weight:
2163.5
Package Type:
Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic [1]. Lariat peptide antiviral [1]. Anti-HIV and anti-HSV (Herpes simplex virus) agent [4-6]. Fsr quorum sensing inhibitor [7-9]. Calmodulin-activated myosin light chain kinase (MLCK) inhibitor [2, 3].
Purity:
>85% (HPLC) (Mixture containing <15% Siamycin II)
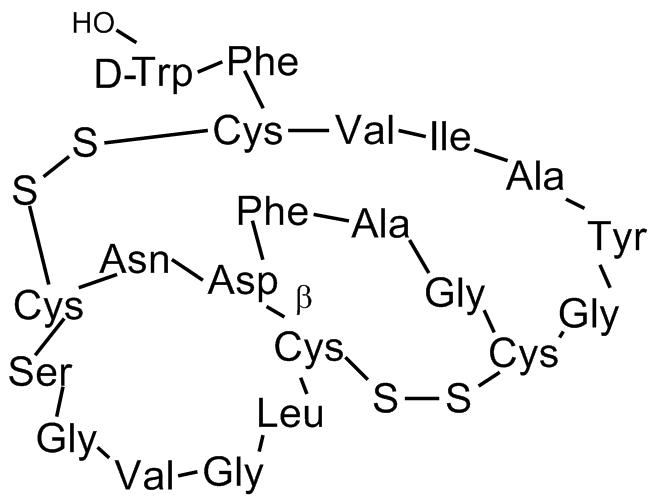
Sequence:
CLGVGSCNDFAGCGYAIVCFW
Signal word:
Warning
SMILES:
[H]OC(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]1([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]2([H])N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]3([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C3([H])[H])C([H])([H])SSC2([H])[H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])O[H])C([H])([H])SSC1([H])[H])C([H])([H])C(=O)N([H])[H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H])C([H])([H])[H])C([H])([H])C1=C([H])C([H])=C(O[H])C([H])=C1[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H])C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Siamycins I and II, new anti-HIV peptides: I. Fermentation, isolation, biological activity and initial characterization: M. Tsunakawa, et al.; J. Antibiot. 48, 433 (1995) | MS-271, a novel inhibitor of calmodulin-activated myosin light chain kinase from Streptomyces sp.--I. Isolation, structural determination and biological properties of MS-271: K. Yano, et al.; Bioorg. Med. Chem. 4, 115 (1996) | MS-271, A Novel Inhibitor of Calmodulin-Activated Myosin Light Chain Kinase from Streptomyces sp.--II. Solution Structure of MS-271: Characteristic Features of the 'Lasso' Structure: R. Katahira, et al.; Bioorg. & Med. Chem. 4, 121 (1996) | FR901724, a novel anti-human immunodeficiency virus (HIV) peptide produced by Streptomyces, shows synergistic antiviral activities with HIV protease inhibitor and 2',3'-dideoxynucleosides: H. Nakashima, et al.; Biol. Pharm. Bull. 19, 405 (1996) | Characterization of siamycin I, a human immunodeficiency virus fusion inhibitor: P.F. Lin, et al.; Antimicrob. Agents Chemother. 40, 133 (1996) | Current Lead Natural Products for the Chemotherapy of Human Immunodeficiency Virus (HIV) Infection: E. De Clercq; Med. Res. Rev. 20, 323 (2000) | Siamycin Attenuates fsr Quorum Sensing Mediated by a Gelatinase Biosynthesis-Activating Pheromone in Enterococcus faecalis: J. Nakayama, et al.; J. Bacteriol. 189, 1358 (2007) | Two-component signal transduction as potential drug targets in pathogenic bacteria: Y. Gotoh, et al.; Curr. Opin. Microbiol. 13, 232 (2010) | Anti-HIV siamycin I directly inhibits autophosphorylation activity of the bacterial FsrC quorum sensor and other ATP-dependent enzyme activities: P. Ma, et al.; FEBS Lett. 585, 2660 (2011)
Related Products
| Product Name | Product Code | Supplier | Aurodox | AG-CN2-0133 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altenusin | AG-CN2-0143 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aspergillimide | AG-CN2-0145 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||