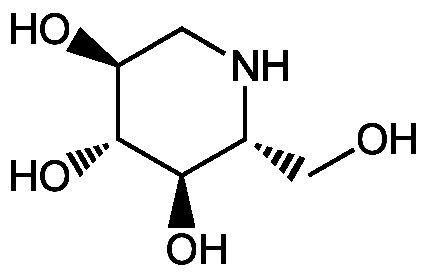
Deoxynojirimycin
| Code | Size | Price |
|---|
| BVT-0112-M001 | 1 mg | £30.00 |
Quantity:
| BVT-0112-M005 | 5 mg | £85.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1,5-Dideoxy-1,5-imino-D-glucitol; Moranoline; DNJ
Appearance:
White to off-white solid.
CAS:
19130-96-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302
InChi:
InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1
InChiKey:
LXBIFEVIBLOUGU-JGWLITMVSA-N
Long Description:
Chemical. CAS: 19130-96-2. Formula: C6H13NO4. MW: 163.2. Isolated from Streptomyces sp. Polyhydroxylated piperidine alkaloid. alpha-Glucosidase I and II inhibitor. Antidiabetic. Antihyperglycaemic activity. Animal feedstuff additive. Antiviral. Shows anti-HIV activity.
MDL:
MFCD00063474
Molecular Formula:
C6H13NO4
Molecular Weight:
163.2
Package Type:
Plastic Vial
Precautions:
P264, P301, P312, P330
Product Description:
Polyhydroxylated piperidine alkaloid. alpha-Glucosidase I and II inhibitor. Antidiabetic. Antihyperglycaemic activity. Glycosylceramidase inhibitor. Animal feedstuff additive. Antiviral. Shows anti-HIV activity.
Purity:
>98% (HPLC, NMR)
Signal word:
Warning
SMILES:
OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or water (10mg/ml).
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Isolation and structure of an unusual cyclic amino alditol from a legume: L.E. Fellows, et al.; Chem. Comm. 22, 977 (1979) | Glucosidase inhibitors from Bacilli: D.D. Schmidt, et al.; Naturwissenschaften 66, 584 (1979) | Glucosiddase inhibitors: structures of deoxynojirimycin and castanospermine: A. Hempel, et al.; J. Med. Chem. 36, 4082 (1993) | Polyhydroxylated alkaloids - natural occurrence and therapeutic applications: A.A. Watson, et al.; Phytochem. 56, 265 (2001) | Small molecule inhibitors of ER alpha-glucosidases are active against multiple hemorrhagic fever viruses: J. Chang, et al.; Antiviral Research 98, 432 (2013) | 1-deoxynojirimycin isolated from a bacillus subtilis stimulates adiponectin and GLUT4 expressions in 3T3-L1 adipocytes: S.-M. Lee, et al.; J. Microb. Biotechn. 23, 637 (2013) | Novel imino sugar alpha-glucosidase inhibitors as antiviral compounds: J.D. Howe, et al.; Bioorg. & Med. Chem. 21, 4831 (2013) | 1-Deoxynojirimycin attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells: E. Shuang, et al.; Exp. Gerontol. 55, 63 (2014) | 1-Deoxynojirimycin isolated from Bacillus subtilis improves hepatic lipid metabolism and mitochondrial function in high-fat-fed mice: H. Do, et al.; Food Chem. Toxicol. 75, 1, (2015) | 1-deoxynojirimycin alleviates insulin resistance via activation of insulin signaling PI3K/AKT pathway in skeletal muscle of db/db mice: Q. Liu, et al.; Molecules 20, 21700 (2015) | Iminosugars as immunomodulating agents: synthesis and biological activities of 1-deoxynojirimycin and related compounds: Q. Li & X.-S. Ye; Isr. J. Chem. 55, 336 (2015) | Iminosugars: the potential of carbohydrate analogs: N. Asano; Carbohydr. Chem. 2016, 279 (2016) | 1-Deoxynojirimycin alleviates liver injury and improves hepatic glucose metabolism in db/db mice: Q. Liu, et al.; Molecules 21, 279/1 (2016) | 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing: K. Gao, et al.; Molecules 21, 1600 (2016) (Review) | Protective mechanism of Rice-derived Lipids and Glucosylceramide in an In Vitro Intestinal Tract Model: S. Yamashita, et al.; J. Agric. Food Chem. 69, 10206 (2021)