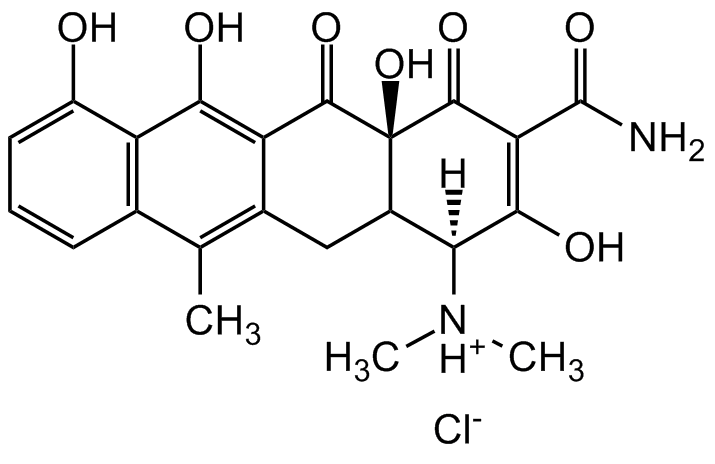
Anhydrotetracycline hydrochloride
| Code | Size | Price |
|---|
| CDX-A0197-M500 | 500 mg | £205.00 |
Quantity:
| CDX-A0197-GG25 | 2.5 g | £792.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20 °C
Images
Documents
Further Information
Alternate Names/Synonyms:
(4S,4aS,12aS)-4-(Dimethylamino)-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-1,4,4a,5,12,12a-hexahydro-2-tetracenecarboxamide hydrochloride (1:1)
Appearance:
Yellow powder.
CAS:
13803-65-1
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C22H22N2O7.ClH/c1-8-9-5-4-6-12(25)13(9)17(26)14-10(8)7-11-16(24(2)3)18(27)15(21(23)30)20(29)22(11,31)19(14)28;/h4-6,11,16,25-27,31H,7H2,1-3H3,(H2,23,30);1H/t11?,16-,22-;/m0./s1
InChiKey:
XBSQEFHDCDFNJU-DXLATOOPSA-N
Long Description:
Chemical. CAS: 13803-65-1. Formula: C22H22N2O7 . HCl. MW: 462.88. Synthetic. A tetracycline derivative exhibiting no antibiotic activity. A useful effector of tetracycline-dependent gene expression in bacterial expression systems.
MDL:
MFCD00151453
Molecular Formula:
C22H22N2O7 . HCl
Molecular Weight:
462.88
Package Type:
Vial
Product Description:
A tetracycline derivative exhibiting no antibiotic activity. A useful effector of tetracycline-dependent gene expression in bacterial expression systems.
Purity:
>98% (HPLC)
SMILES:
[Cl-].[H][C@@]1(C2CC3=C(C)C4=C(C(O)=CC=C4)C(O)=C3C(=O)[C@]2(O)C(=O)C(C(N)=O)=C1O)[NH+](C)C
Solubility Chemicals:
Soluble in water or acetonitrile.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) T. Sokoloski, et al.; J. Pharm. Sci. 66, 1159 (1977) | (2) L. Paemen, et al.; Biochem. Pharmacol. 5, 105 (1996) | Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance: A.J. Gasparrini, et al.; Commun. Biol. 3, 241 (2020) | Heterologous Catalysis of the Final Steps of Tetracycline Biosynthesis by Saccharomyces cerevisiae: E. Herbst, et al.; ACS Chem. Biol. ahead of print (2021)
Related Products
| Product Name | Product Code | Supplier |
|---|