DAN
| Code | Size | Price |
|---|
| CDX-D0062-G001 | 1 g | £159.00 |
Quantity:
| CDX-D0062-G005 | 5 g | £517.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4 °C
Images
Documents
Further Information
Alternate Names/Synonyms:
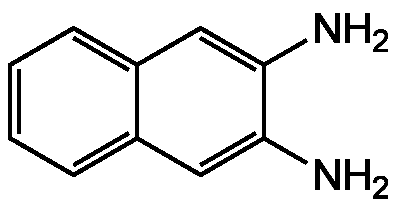
2,3-Diaminonaphthalene
Appearance:
Off-white to light brown solid.
CAS:
771-97-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H315, H319, H335, H350
InChi:
InChI=1S/C10H10N2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6H,11-12H2
InChiKey:
XTBLDMQMUSHDEN-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 771-97-1. Formula: C10H10N2. MW: 158.2. Synthetic. The 2,3-diaminonaphthalene (DAN) assay is routinely used in the determination of nitrite/nitrate levels in biological fluids and cellular extracts as one indicator of nitric oxide activity. The assay, as reported by Misko, uses the reaction of DAN with NO2- under acidic conditions to form a detectable fluorescent naphthotriazole. The reaction proceeds at acidic pH at room temperature. Fluorescence is monitored following the addition of NaOH, which raises pH, resulting in lower background and increased sensitivity (Ex/Em: ~365/415nm). However, detection at 450nm is recommended to avoid fluorescent blanks and increase sensitivity. The fluorescent background of DAN is low for maximum sensitivity. Detection limits of NO achieved by this method are in the high-pM range. 2,3-Diaminonaphtaline (DAN) is used as a derivatisation-reagent for the determination of selenium at low detection limits. The 4,5-benzopiaselenol-complex can be quantified by photometry or GC.
MDL:
MFCD00004116
Molecular Formula:
C10H10N2
Molecular Weight:
158.2
Package Type:
Vial
Precautions:
P201, P261, P305, P351, P338, P308, P313
Product Description:
The 2,3-diaminonaphthalene (DAN) assay is routinely used in the determination of nitrite/nitrate levels in biological fluids and cellular extracts as one indicator of nitric oxide activity. The assay, as reported by Misko, uses the reaction of DAN with NO2- under acidic conditions to form a detectable fluorescent naphthotriazole. The reaction proceeds at acidic pH at room temperature. Fluorescence is monitored following the addition of NaOH, which raises pH, resulting in lower background and increased sensitivity (Ex/Em: ~365/415nm). However, detection at 450nm is recommended to avoid fluorescent blanks and increase sensitivity. The fluorescent background of DAN is low for maximum sensitivity. Detection limits of NO achieved by this method are in the high-pM range. 2,3-Diaminonaphtaline (DAN) is used as a derivatisation-reagent for the determination of selenium at low detection limits. The 4,5-benzopiaselenol-complex can be quantified by photometry or GC.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
NC1=CC2=CC=CC=C2C=C1N
Solubility Chemicals:
Soluble in DMSO, DMF or pyridine.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) J. Pedro et al.; Anal. Chim. Acta 516(1-2), 229 (2004) | (2) T. Nagano et al.; Biol. Pharm. Bull. 21(12), 1247 (1998) | (3) W.C. Hawkes et al.; Anal. Biochem. 241(2), 206 (1996) | (4) R.F. Bayfield et al.; Anal. Biochem. 144(2), 569 (1995) | (5) P. Damiani et al.; Talanta 33(8), 649 (1986)
Related Products
| Product Name | Product Code | Supplier | DPM | CDX-D0049 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PyTC2 | CDX-D0050 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6,8-Difluoro-7-hydroxy-4-methylcumarin | CDX-D0059 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCFDA | CDX-D0063 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3,8-Diamino-5-methyl-6-phenylphenanthridinium bromide | CDX-D0065 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||