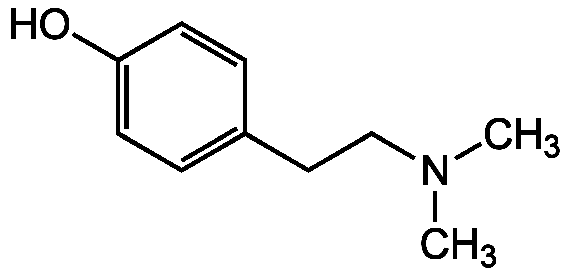
Hordenin
| Code | Size | Price |
|---|
| CDX-H0099-G001 | 1 g | £102.00 |
Quantity:
| CDX-H0099-G005 | 5 g | £188.00 |
Quantity:
| CDX-H0099-G050 | 50 g | £1,226.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4 °C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-(4-Hydroxyphenyl)-N,N-dimethylethylamine, 4-(2-Dimethylaminoethyl)phenol, N,N-Dimethyltyramine, p-Hydroxy-N,N-dimethylphenethylamine, Anhaline, Cactine, Eremursin, Peyocactine
Appearance:
Beige to brown solid.
CAS:
539-15-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H317, H319
InChi:
InChI=1S/C10H15NO/c1-11(2)8-7-9-3-5-10(12)6-4-9/h3-6,12H,7-8H2,1-2H3
InChiKey:
KUBCEEMXQZUPDQ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 539-15-1. Formula: C10H15NO. MW: 165.23. Synthetic. Hordenine is a phenethylamine type alkaloid found naturally in various plants such as barley and cacti. Hordenine may be used as an analytical reference material and in research on the activities of phenethylamine type alkaloids. Quorum-sensing inhibitor (QSI).
MDL:
MFCD00051462
Molecular Formula:
C10H15NO
Molecular Weight:
165.23
Package Type:
Vial
Precautions:
P280, P305, P351, P338
Product Description:
Hordenine is a phenethylamine type alkaloid found naturally in various plants such as barley and cacti. Hordenine may be used as an analytical reference material and in research on the activities of phenethylamine type alkaloids. Quorum-sensing inhibitor (QSI).
Purity:
>97% (HPLC)
Signal word:
Warning
SMILES:
CN(C)CCC1=CC=C(O)C=C1
Solubility Chemicals:
Soluble in DMF, acetonitrile or chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
At a supra-physiological concentration, human sexual hormones act as quorum-sensing inhibitors: A. Beury-Cirou, et al.; PLoS One 8, e83564 (2013)