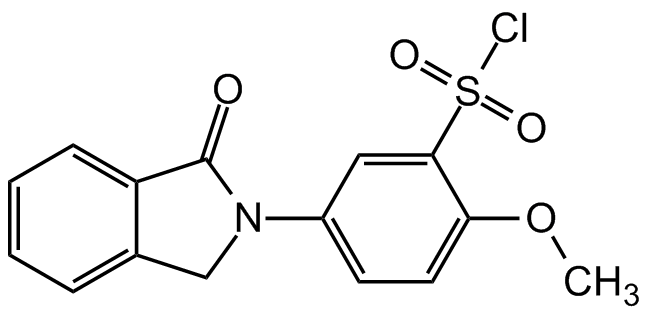
M-Phisyl-chloride
| Code | Size | Price |
|---|
| CDX-M0004-M100 | 100 mg | £158.00 |
Quantity:
| CDX-M0004-M500 | 500 mg | £500.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20 °C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Methoxy-5-(N-phthalimidinyl)benzenesulfonyl chloride
Appearance:
Solid.
CAS:
126565-42-2
Class:
8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05
Handling Advice:
Protect from light and moisture.
Hazards:
H314
InChi:
InChI=1S/C15H12ClNO4S/c1-21-13-7-6-11(8-14(13)22(16,19)20)17-9-10-4-2-3-5-12(10)15(17)18/h2-8H,9H2,1H3
InChiKey:
ABWFLHUIHQOEBX-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 126565-42-2. Formula: C15H12ClNO4S. MW: 337.78. Synthetic. It is known that phenol and p-cresol are promoters of skin tumors and also urinary excretion of phenol and p-cresol is increased by exposure to benzene, which has hemopoietic toxicity, and toluene, respectively. Therefore, the determination of phenol and p-cresol is important in clinical chemistry and toxicology. Because phenol and p-cresol are present in urine as sulfate or glucuronide conjugates, they are hydrolyzed enzymatically or with acid, followed by distillation or extraction with organic solvent prior to measurement by GC or HPLC. However, these GC and HPLC methods require relatively large sample volumes (more than 1 ml) because of their insufficient sensitivities for measuring small amounts of phenol and p-cresol. In the general, the application of fluorescent labeling techniques to HPLC result in a sensitive and selective method. Some acyl chlorides are known to react with phenolic hydroxy group to form esters. It is known that 4-(N-phthalimidinyl)benzensulfonyl chloride Phisyl-Cl7, having sulfonyl chloride, reacted with phenol to give the corresponding highly fluorescent sulfonyl ester. The sensitivity of Physil-Cl was compared with Dansyl-Cl and DPS-Cl, which are analogous to Physil-Cl as fluorescent labeling reagents having sulfonyl chloride group. The labeling reaction of phenol with Dansyl-Cl and DPS-Cl were carried out according to the method of Ruiter et al.5 and the peaks were detected at the each fluorescence maxima wavelength (Ex350nm a Em550nm for Dansyl-Cl, and Ex320nm a Em386nm for DPS-Cl). Peak height of Phisyl derivative was higher about 85-and 7,3-fold than thosse of Dansyl derivative and DPS derivative, respectively. Among these three reagents, Phisyl-Cl was the most sensitive.
MDL:
MFCD16657087
Molecular Formula:
C15H12ClNO4S
Molecular Weight:
337.78
Package Type:
Vial
PG:
III
Precautions:
P280, P305, P351, P338, P310
Product Description:
It is known that phenol and p-cresol are promoters of skin tumors and also urinary excretion of phenol and p-cresol is increased by exposure to benzene, which has hemopoietic toxicity, and toluene, respectively. Therefore, the determination of phenol and p-cresol is important in clinical chemistry and toxicology. Because phenol and p-cresol are present in urine as sulfate or glucuronide conjugates, they are hydrolyzed enzymatically or with acid, followed by distillation or extraction with organic solvent prior to measurement by GC or HPLC. However, these GC and HPLC methods require relatively large sample volumes (more than 1 ml) because of their insufficient sensitivities for measuring small amounts of phenol and p-cresol. In the general, the application of fluorescent labeling techniques to HPLC result in a sensitive and selective method. Some acyl chlorides are known to react with phenolic hydroxy group to form esters. It is known that 4-(N-phthalimidinyl)benzensulfonyl chloride Phisyl-Cl7, having sulfonyl chloride, reacted with phenol to give the corresponding highly fluorescent sulfonyl ester. The sensitivity of Physil-Cl was compared with Dansyl-Cl and DPS-Cl, which are analogous to Physil-Cl as fluorescent labeling reagents having sulfonyl chloride group. The labeling reaction of phenol with Dansyl-Cl and DPS-Cl were carried out according to the method of Ruiter et al.5 and the peaks were detected at the each fluorescence maxima wavelength (Ex350nm a Em550nm for Dansyl-Cl, and Ex320nm a Em386nm for DPS-Cl). Peak height of Phisyl derivative was higher about 85-and 7,3-fold than thosse of Dansyl derivative and DPS derivative, respectively. Among these three reagents, Phisyl-Cl was the most sensitive.
Purity:
>97% (Elemental Analysis)
Signal word:
Danger
SMILES:
COC1=CC=C(C=C1S(Cl)(=O)=O)N1CC2=CC=CC=C2C1=O
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Synthetic.
Transportation:
Excepted Quantity
UN Nummer:
UN 3261
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) Y. Tsuruta et al.; J. Chromatogr. 8, 178 (1994) | (2) K. Imai et al.; Biomed. Chromatogr. 8, 107 (1994) | (3) C. Ruiter et al.; Anal. Chem 60, 666 (1988) | (4) R.M. Cassidy et al.; J. Chromatogr. Sci. 12, 85 (1974)