Me6BIO
| Code | Size | Price |
|---|
| AG-MR-C0021-M005 | 5 mg | £100.00 |
Quantity:
| AG-MR-C0021-M025 | 25 mg | £325.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
MeBIO; 1-Methyl-6-bromo-indirubin-3'-oxime
Appearance:
Dark red solid.
CAS:
667463-95-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
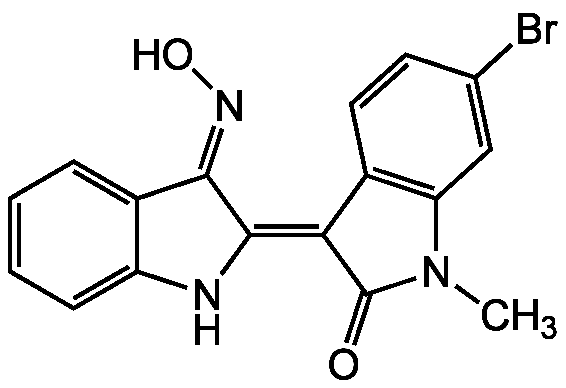
InChI=1S/C17H12BrN3O2/c1-21-13-8-9(18)6-7-11(13)14(17(21)22)16-15(20-23)10-4-2-3-5-12(10)19-16/h2-8,19,23H,1H3/b16-14-,20-15+
InChiKey:
JUKQKURGZYNHFN-NVVUQHMZSA-N
Long Description:
Chemical. CAS: 667463-95-8. Formula: C17H12BrN3O2. MW: 370.2. N-methylated inactive control analog of 6-bromoindirubin-3'-oxime (6BIO) (Prod. No. MR-C0019). Displays minimal activity against CDK1/Cyclin B, GSK-3 alpha/beta and CDK5/p25. Aryl hydrocarbon receptor (AhR) agonist; causes redistribution of AhR to the nucleus.
MDL:
MFCD09037537
Molecular Formula:
C17H12BrN3O2
Molecular Weight:
370.2
Package Type:
Vial
Precautions:
P261, P271, P280, P312
Product Description:
N-methylated inactive control analog of 6-bromoindirubin-3'-oxime (6BIO) (Prod. No. MR-C0019). Displays minimal activity against CDK1/Cyclin B, GSK-3 alpha/beta and CDK5/p25. Aryl hydrocarbon receptor (AhR) agonist; causes redistribution of AhR to the nucleus.
Purity:
>95% (NMR)
Signal word:
Warning
SMILES:
CN1C(=O)C(=C2/NC3=CC=CC=C3/C/2=NO)C2=C1C=C(Br)C=C2
Solubility Chemicals:
Soluble in DMSO or ethanol.
Transportation:
Non-hazardous
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
GSK-3-selective inhibitors derived from Tyrian purple indirubins: L. Meijer, et al.; Chem. Biol. 10, 1255 (2003) | Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases: P. Polychronopoulos, et al.; J. Med. Chem. 47, 935 (2004) | Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the antiproliferative effects of indirubins: M. Knockaert, et al.; Oncogene 23, 4400 (2004) | The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes: A.S. Tseng, et al.; Chem. Biol. 13, 957 (2006) | Indirubin, the red shade of indigo: L. Meijer, et al. (Editors): In ?Life in Progress?, Station Biologique, Roscoff, 297 pp. (2006) | Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments: S.P. Williams, et al.; Cancer Res. 71, 5374 (2011) | Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells: C.R. McClain, et al.; J. Neurosci. 32, 15066 (2012)
Related Products
| Product Name | Product Code | Supplier | Indole-3-carbinol | AG-CR1-3637 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6BIO | AG-MR-C0019 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7BIO | AG-MR-C0020 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||