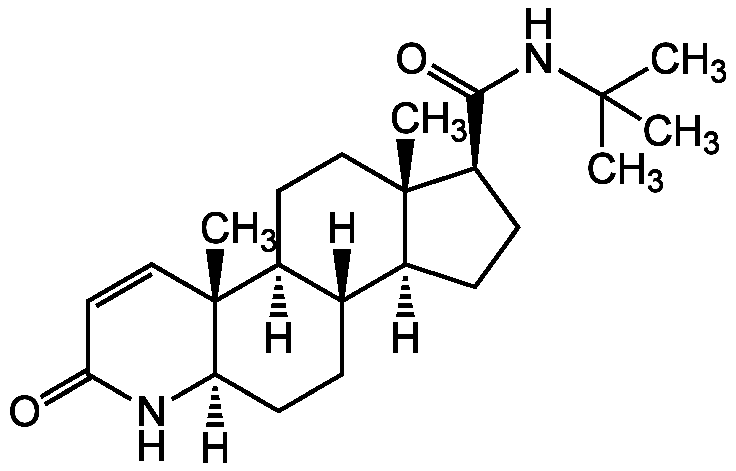
Finasteride
| Code | Size | Price |
|---|
| AG-CR1-3589-M025 | 25 mg | £25.00 |
Quantity:
| AG-CR1-3589-M100 | 100 mg | £45.00 |
Quantity:
| AG-CR1-3589-M500 | 500 mg | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
MK 906; L-652,931; BRN 4269024
Appearance:
White to off-white solid.
CAS:
98319-26-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1
InChiKey:
DBEPLOCGEIEOCV-WSBQPABSSA-N
Long Description:
Chemical. CAS: 98319-26-7. Formula: C23H36N2O2. MW: 372.5. Potent, specific and competitive inhibitor of type II 5alpha-reductase (enzyme which converts testosterone to the more potent 5alpha-dihydrotestosterone). Anticancer compound. Apoptosis modulator. Enhances the action of GABA at GABA(A) receptors, which leads to neurological implications. Inhibit testosterone-induced type I procollagen and TGF-beta1 expression in human scalp dermal fibroblasts in a model of androgenic alopecia. Drug for the treatment of male androgenetic alopecia. Potential role in neuropsychiatric disorders.
MDL:
MFCD00869737
Molecular Formula:
C23H36N2O2
Molecular Weight:
372.5
Package Type:
Vial
Precautions:
P270, P301, P312, P330
Product Description:
Potent, specific and competitive inhibitor of type II 5alpha-reductase (enzyme which converts testosterone to the more potent 5alpha-dihydrotestosterone) [1, 3]. Anticancer compound [2, 6, 7]. Apoptosis modulator [2, 6]. Enhances the action of GABA at GABA(A) receptors, which leads to neurological implications [4]. Inhibit testosterone-induced type I procollagen and TGF-beta1 expression in human scalp dermal fibroblasts in a model of androgenic alopecia [5]. Drug for the treatment of male androgenetic alopecia [8]. Potential role in neuropsychiatric disorders [9]. Shown to reduce L-DOPA-induced dyskinesia in rodent models for Parkinson?s disease [10].
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])NC(=O)C=C[C@]12C
Solubility Chemicals:
Soluble in DMSO, DMF, ethanol or methanol. Sparingly soluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Finasteride: the first 5 alpha-reductase inhibitor: S.L. Sudduth & M.J. Koronkowski; Pharmacotherapy 13, 309 (1993) | Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins: H. Huynh; Int. J. Oncol. 20, 1297 (2002) | Steroid 5alpha-reductase inhibitors: R. Flores, et al.; Mini Rev. Med. Chem. 3, 225 (2003) | A new look at the 5alpha-reductase inhibitor finasteride: D.A. Finn, et al.; CNS Drug Rev. 12, 53 (2006) | Perifollicular fibrosis: Pathogenetic role in androgenetic alopecia; H.G. Yoo, et al.; Biol. Pharm. Bull. 29, 1246 (2006) | Finasteride induces apoptosis via Bcl-2, Bcl-xL, Bax and caspase-3 proteins in LNCaP human prostate cancer cell line: J.M. Golbano, et al.; Int. J. Oncol. 32, 919 (2008) | Molecular profiles of finasteride effects on prostate carcinogenesis: Cancer Prev. Res. 2, 518 (2009) | Male androgenetic alopecia: D. Rathnayake & R. Sinclair; Expert Opin. Pharmacother. 11, 1295 (2010) | Steroid 5alpha-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders: S. Paba, et al.; Curr. Pharm. Des. 17, 151 (2011) | 5alpha-reductase inhibitors dampen L-DOPA-induced dyskinesia via normalization of dopamine D1-receptor signaling pathway and D1-D3 receptor interaction: S. Fanni, et al.; Neurobiol. Dis. 121, 120 (2018)