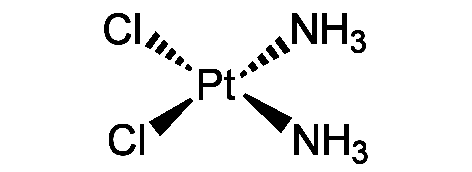
Cisplatin
| Code | Size | Price |
|---|
| AG-CR1-3590-M050 | 50 mg | £40.00 |
Quantity:
| AG-CR1-3590-M250 | 250 mg | £80.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
cis-Diamminedichloroplatinum (II); Neoplatin; CDDP; cis-DDP; NSC119875; NK 801; TR170
Appearance:
Yellow solid.
CAS:
15663-27-1
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS06
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H300, H315, H318
InChi:
InChI=1S/2ClH.2H3N.Pt/h2*1H;2*1H3;/q;;;;+2/p-2
InChiKey:
LXZZYRPGZAFOLE-UHFFFAOYSA-L
Long Description:
Chemical. CAS: 15663-27-1. Formula: Cl2H6N2Pt. MW: 300. Potent platinum-based antineoplastic agent. Anticancer compound. Forms inter- and intrastrand DNA adducts/crosslinks, consequently blocking DNA replication and transcription and inducing cell death. Nephrotoxic when used in vivo. Potent chemoimmunotherapeutic. Stimulates the immune responses by activating macrophages and other cells of the immune system. Apoptosis inducer via p53-dependent and -independent mechanisms. Induces apoptosis through caspase-3 activation and XIAP expression inhibition. Targets other proteins and enzymes. Non-competitive NHE-1 inhibitor, an archetypal, membrane-bound mechanosensitive sodium-hydrogen ion transporter.
MDL:
MFCD00011623
Molecular Formula:
Cl2H6N2Pt
Molecular Weight:
300
Other data:
Note: In aqueous solution cis-trans isomerization of cisplatin occurs. Isomerization is increased at elevated temperatures. Stability of cisplatin in aqueous solutions was enhanced by increasing the sodium chloride (NaCl) concentration to 0.9% and was adversely affected in alkaline solutions such as sodium bicarbonate solutions. Further, even though soluble in DMSO, we do not recommend to dissolve cisplatin in DMSO. DMSO inserts itself into the ligand. It is recommended to prepare all solutions fresh and protected from light.
Package Type:
Vial
PG:
III
Precautions:
P261, P280, P301, P310, P302, P352, P305, P351, P338
Product Description:
Potent platinum-based antineoplastic agent [1, 10]. Anticancer compound [1]. Forms inter- and intrastrand DNA adducts/crosslinks, consequently blocking DNA replication and transcription and inducing cell death [2, 12, 13, 15]. Nephrotoxic when used in vivo [3]. Potent chemoimmunotherapeutic. Stimulates the immune responses by activating macrophages and other cells of the immune system [4, 6]. Apoptosis inducer via p53-dependent and -independent mechanisms [5, 7, 8]. Induces apoptosis through caspase-3 activation and XIAP expression inhibition [9, 14]. Targets other proteins and enzymes [10]. Non-competitive NHE-1 inhibitor, an archetypal, membrane-bound mechanosensitive sodium-hydrogen ion transporter [16].
Purity:
>98%
Signal word:
Danger
SMILES:
N[Pt](N)(Cl)Cl
Solubility Chemicals:
Soluble in DMF or DMSO. Sparingly soluble in water. Insoluble in ethanol.
Transportation:
Excepted Quantity
UN Nummer:
UN 3288
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Antitumor action of cis-dichlorobis(methylamine)platinum(II): G.R. Gale, et al.; J. Natl. Cancer Inst. 51, 1227 (1973) | Preliminary characterization of the adducts formed between the antitumor compounds cis-Pt(NH3)2Cl2 and DNA: N.P. Johnson; BBRC 104, 1394 (1982) | Plasma platinum levels: relationship to cisplatin dose and nephrotoxicity: A.B. Campbell, et al.; Cancer Treat. Rep. 67, 169 (1983) | Immune response of mice exposed to cis-diamminedichloroplatinum: O. Bagasra, et al.; Cancer Immunol. Immunother. 19, 142 (1985) | Cisplatin induces a persistent activation of JNK that is related to cell death: I. Sanchez-Perez, et al.; Oncogene 16, 533 (1998) | Antigen presentation by cisplatin-activated macrophages: role of soluble factor(s) and second messengers: R.A. Singh & A. Sodhi; Immunol. Cell Biol. 76, 513 (1998) | Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of the Fas/Fas ligand system: M.S. Razzaque, et al.; Histochem. Cell Biol. 111, 359 (1999) | The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage: J.G. Gong, et al.; Nature 399, 806 (1999) | Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcoma: K. Seki, et al.; Cancer Chemother. Pharmacol. 45, 199 (2000) | What is the "best" platinum: cisplatin, carboplatin, or oxaliplatin? J. Lokich; Cancer Invest. 19, 756 (2001) | Biomolecular targets for platinum antitumor drugs: R.N. Bose; Mini Rev. Med. Chem. 2, 103 (2002) | Cisplatin: mode of cytotoxic action and molecular basis of resistance: Z.H. Siddik; Oncogene 22, 7265 (2003) | New clues for platinum antitumor chemistry: Kinetically controlled metal binding to DNA: J. Reedijk; PNAS 100, 3611 (2003) | Cisplatin inhibits the expression of X-linked inhibitor of apoptosis protein in human LNCaP cells: T. Nomura, et al.; Urol. Oncol. 22, 453 (2004) | Inhibition of transcription by platinum antitumor compounds: R.C. Todd & S.J. Lippard; Metallomics 1, 280 (2009) | Nongenomic effects of cisplatin: acute inhibition of mechanosensitive transporters and channels without actin remodeling: N. Milosavljevic, et al.; Cancer Res. 70, 7514 (2010) | RANKL signaling sustains primary tumor growth in genetically engineered mouse models of lung adenocarcinoma: J. Faget, et al.; J. Thoracic Oncol. 13, 387 (2017) | GADD45alpha-targeted suicide gene therapy driven by synthetic CArG promoter E9NS sensitizes NSCLC cells to cisplatin, resveratrol, and radiation regardless of p53 status: Q. Shi, et al.; Onco Targets Ther. 12, 3161 (2019) | Single-event tandem ICP-mass spectrometry for the quantification of chemotherapeutic drug-derived Pt and endogenous elements in individual human cells: T. Liu, et al.; Analyt. Chim. Acta 1177, 338797 (2021)