Ageladine A . TFA
| Code | Size | Price |
|---|
| AG-CMA-1001-C200 | 200 ug | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
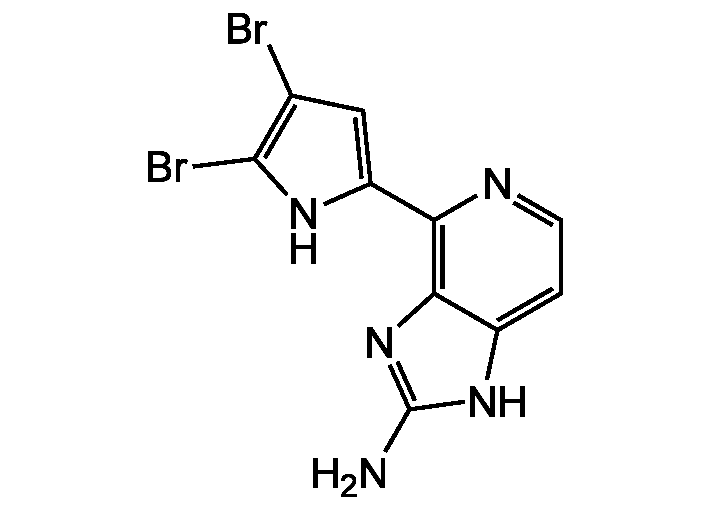
(4-(4,5-Dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]-pyridin-2-amine) . TFA
Appearance:
Brown solid.
CAS:
950766-21-9 | 643020-13-7 (free base)
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.Protect from light when in solution.
InChi:
InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17)
InChiKey:
QAKGJAQGTQLMFN-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 950766-21-9 | 643020-13-7 (free base). Formula: C10H7Br2N5 . C2HF3O2. MW: 357.0 . 114.0. Synthetic. Originally isolated from Agelas nakamurai. Non-toxic, pH-sensitive fluorescent dye (blue-green range) for live imaging of pH alteration in acidic organelles, vesicles, cells, tissue and even small whole animals over several days without side effects. Stronger fluorescent intensity under acidic conditions and barely detectable in alkaline solutions (wide range from pH 4 to pH 8). Highly cell/membrane permeable. Trapped within the cells and acidic organelles through hydrophobic interactions with the inner side of the membranes. Barely metabolized and long-term stable, thus slow photobleaching. Angiogenesis inhibitor. Inhibitor of matrix metalloproteinases MMP-1,-2,-8,-9,-12,-13. TYK2, DYRK2,Dyrk1A, YSK4 and RPS6KA1/2 inhibitor.
MDL:
MFCD28899026
Molecular Formula:
C10H7Br2N5 . C2HF3O2
Molecular Weight:
357.0 . 114.0
Other data:
Fluorescence specifications: Excitation 325 - 415nm; maximum at 370nm Emission 415 - 500nm; maximum at 415nm Prepare a 1mM stock solution in MeOH or DMSO:Dissolve 0.425ml methanol into the provided 0.2mg vial of Ageladine A, mix gently until the compound is completely dissolved. The solution should show a homogenous yellow colour. Use fresh preparations or aliquot and freeze immediately. Ageladine A is light sensitive, avoid longer exposition to direct sun light or other intensive light sources. Avoid freeze/thaw cycles of solutions.
Package Type:
Vial
Product Description:
Non-toxic, pH-sensitive fluorescent dye (blue-green range) for live imaging of pH alteration in acidic organelles, vesicles, cells, tissue and even small whole animals over several days without side effects [2,4,5,7-9]. Stronger fluorescent intensity under acidic conditions and barely detectable in alkaline solutions (wide range from pH 4 to pH 8) [2]. Highly cell/membrane permeable. Trapped within the cells and acidic organelles through hydrophobic interactions with the inner side of the membranes [2]. Barely metabolized and long-term stable, thus slow photobleaching [9]. Angiogenesis inhibitor [1,3,6]. Inhibitor of matrix metalloproteinases MMP-1,-2,-8,-9,-12,-13 [1, 3-6]. TYK2, DYRK2,Dyrk1A, YSK4 and RPS6KA1/2 inhibitor [3,5,6].
Purity:
>98% (NMR)
SMILES:
NC1=NC2=C(N=CC=C2N1)C1=CC(Br)=C(Br)N1
Solubility Chemicals:
Soluble in DMSO or methanol.
Source / Host:
Synthetic. Originally isolated from Agelas nakamurai.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
12171500
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C. Stock solutions are stable for at least 3 months when stored at -20°C.
References
Ageladine A: An antiangiogic matrixmetalloproteinase inhibitor from the marine sponge Agelas nakamurai: M. Fujita, et al.; JACS 125, 1570 (2003) | Ageladine A, a pyrrole-imidazole alkaloid from marine sponges, is a pH sensitive membrane permeable dye: U. Bickmeyer, et al.; BBRC 373, 419 (2008) | Synthesis and anticancer activities of ageladine A and structural analogs: Y. Ma, et al.; Bioorg. Med. Chem. Lett. 20, 83 (2010) | Tracking of fast moving neuronal vesicles with ageladine A: U. Bickmeyer, et al.; BBRC 402, 489 (2010) | A one-pot synthesis and biological activity of ageladine A and analogues: S.R. Shengule, et al.; Med. Chem. 54, 2492 (2011) | Synthesis and matrix metalloproteinase-12 inhibitory activity of ageladine A analogs: N. Ando & S. Terashima; Chem. Pharm. Bull. 59, 579 (2011) | The alkaloid Ageladine A, originally isolated from marine sponges, used for pH-sensitive imaging of transparent marine animals: U. Bickmeyer; Mar. Drugs 10, 223 (2012) | Incorporated nematocysts in Aeolidiella stephanieae (Gastropoda, Opisthobranchia, Aeolidoidea) mature by acidification shown by the pH sensitive fluorescing alkaloid Ageladine A: D. Obermann, et al.; Toxicon 60, 1108 (2012) | Reporter dyes demonstrate functional expression of multidrug resistance proteins in the marine flatworm Macrostomum lignano: The sponge-derived dye ageladine A is not a substrate of these transporters: K. Tietje, et al.; Mar. Drugs 11, 3951 (2013) | Marine-derived angiogenesis inhibitors for cancer therapy: Y.Q. Wang & Z.H. Miao; Mar. Drugs 11, 903 (2013) | The physiological response of the marine platyhelminth Macrostomum lignano to different environmental oxygen concentrations: G.A. Rivera-Ingraham, et al.; J. Exp. Biol. 216, 2741 (2013) | The chemically synthesized ageladine A-derivative LysoGlow84 stains lysosomes in viable mammalian brain cells and specific structures in the marine flatworm Macrostomum lignano: T. Mordhorst, et al.; Mar. Drugs 13, 920 (2015)