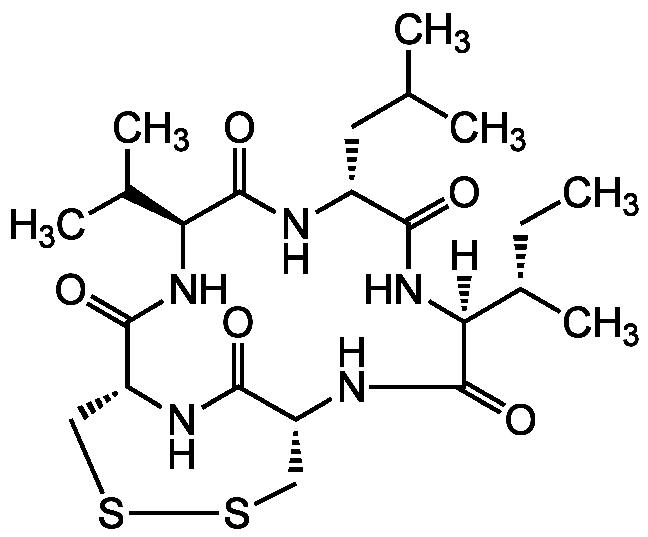
Malformin A1
Product Code:
AG-CN2-0169
AG-CN2-0169
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0169-C250 | 250 ug | £100.00 |
Quantity:
| AG-CN2-0169-M001 | 1 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Malformin A; Cyclic(D-cysteinyl-D-cysteinyl-L-valyl-D-leucyl-L-isoleucyl)cyclic(1-2)-disulfide
Appearance:
White solid.
CAS:
3022-92-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C24H40N4O5S2/c1-7-14(6)20-24(33)26-16-10-34-35-11-17(25-22(16)31)23(32)27-19(13(4)5)18(29)9-15(8-12(2)3)21(30)28-20/h12-17,19-20H,7-11H2,1-6H3,(H,25,31)(H,26,33)(H,27,32)(H,28,30)/t14-,15-,16+,17+,19-,20-/m0/s1
InChiKey:
BEZOVOWRERTMCU-VEXWFWOFSA-N
Long Description:
Chemical. CAS: 3022-92-2. Formula: C23H39N5O5S2. MW: 529.7. Synthetic. Originally isolated from Aspergillus niger. Peptide antibiotic. Antibcaterial. Plant growth stimulator. Induces root curvature and malformation in plants. Mycotoxin. Prevents interleukin-1 (IL-1) induced endothelial changes by inhibition of protein synthesis. Inhibitor of interleukin-1 beta (IL1 beta) binding to its receptor. Enhancer of cellular fibrinolytic activity. Disrupts the cell cycle at the G2 checkpoint of cancer cells, leading to sensitization of the cancer cells to anti-cancer reagents. Anticancer compound. Cytotoxic against several cancer cell lines. Antimalarial and antitrypanosomal. Inhibitor of BRAF-mutated melanoma cell lines.
MDL:
MFCD00133524
Molecular Formula:
C23H39N5O5S2
Molecular Weight:
529.7
Package Type:
Vial
Precautions:
P270, P301, P312, P330
Product Description:
Peptide antibiotic. Antibcaterial [1]. Plant growth stimulator [2]. Induces root curvature and malformation in plants [3]. Mycotoxin [4]. Prevents interleukin-1 (IL-1) induced endothelial changes by inhibition of protein synthesis [5]. Inhibitor of interleukin-1 beta (IL1 beta) binding to its receptor [6]. Enhancer of cellular fibrinolytic activity [7, 12]. Disrupts the cell cycle at the G2 checkpoint of cancer cells, leading to sensitization of the cancer cells to anti-cancer reagents [8]. Anticancer compound. Cytotoxic against several cancer cell lines [9]. Antimalarial and antitrypanosomal [10]. Inhibitor of BRAF-mutated melanoma cell lines [11].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]1(NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H]2CSSC[C@@H](NC1=O)C(=O)N2)C(C)C)[C@@H](C)CC
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Synthetic. Originally isolated from Aspergillus niger.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Antibiotic properties of malformin: S. Suda & R.W. Curtis; Appl. Microbiol. 14, 475 (1966) | Stimulation of plant growth by malformin A: W.W. John & R.W. Curtis; Experientia 30, 1392 (1974) | Structure and synthesis of malformin A1: M. Bodanszky & G.L. Stahl; Bioorg. Chem. 4, 93 (1975) | Mycotoxins from mold fungi-weapons of uninvited fellow-boarders of man and animal: structures, biological activity, biosynthesis, and precautions: B. Franck; Angew. Chem. Int. Ed. 23, 493 (1984) | Malformin A prevents IL-1 induced endothelial changes by inhibition of protein synthesis: P.G. Bannon, et al. Thromb. Haemost. 72, 482 (1994) | Malformin-A1 inhibits the binding of interleukin-1 beta (IL1 beta) and suppresses the expression of tissue factor in human endothelial cells and monocytes: J.M. Herbert, et al.; Biochem. Pharmacol. 48, 1211 (1994) | Enhancement of fibrinolytic activity of U937 cells by malformin A1: Y. Koizumi & K. Hasumi; J. Antibiot. 55, 78 (2002) | Fungal malformins inhibit bleomycin-induced G2 checkpoint in Jurkat cells: K. Hagimori, et al.; Biol. Pharm. Bull. 30, 1379 (2007) | Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. J. Zhan, et al.; Phytochem. 68, 368 (2007) | Solid-phase synthesis and biological activity of malformin C and its derivatives: Y. Kojima, et al.; J. Antibiot. (Tokyo) 62, 681 (2009) | A cell-based screening to detect inhibitors of BRAF signaling pathway: Y. Asami, et al.; J. Antibiot. 62, 105 (2009) | Fibrinolytic activation promoted by the cyclopentapeptide malformin: involvement of cytoskeletal reorganization: Y. Koizumi, et al.; Biol. Pharm. Bull. 34, 1426 (2011) | Malformin A1 treatment alters invasive and oncogenic phenotypes of human colorectal cancer cells through stimulation of the p38 signaling pathway: SY. Park, et al.; Int. J. Oncol. 51, 959 (2017)