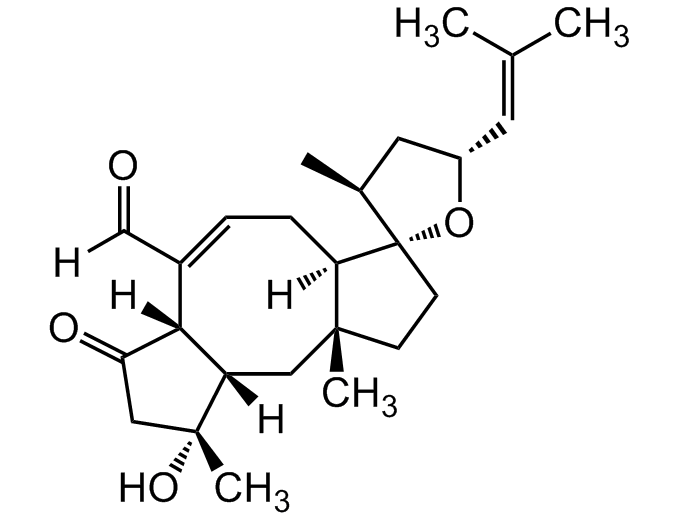
Ophiobolin A
Product Code: AG-CN2-0431
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0431-C100 | 100 ug | £120.00 |
Quantity:
| AG-CN2-0431-M001 | 1 mg | £320.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Cochliobolin A; Ophiobalin; NSC 114340
Appearance:
White solid.
CAS:
06/05/4611
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep under inert gas.Protect from light.Protect from light when in solution.
Hazards:
H301 , H332
InChi:
InChI=1S/C25H36O4/c1-15(2)10-18-11-16(3)25(29-18)9-8-23(4)12-19-22(20(27)13-24(19,5)28)17(14-26)6-7-21(23)25/h6,10,14,16,18-19,21-22,28H,7-9,11-13H2,1-5H3/b17-6-/t16-,18-,19-,21+,22+,23+,24+,25-/m0/s1
InChiKey:
MWYYLZRWWNBROW-BDZRSQQBSA-N
Long Description:
Chemical. CAS: 4611-05-6. Formula: C25H36O4. MW: 400.6. Isolated from Bipolaris leersia. Cell permeable, irreversible calmodulin antagonist. Acts by covalently binding to a lysine-rich inhibitory site. Inhibits by blocking the activation of the Ca2+/calmodulin-dependent phosphodiesterase. Herbicidal mycotoxin. Phytotoxic, antifungal, antibacterial and nematocidal compound. Anticancer compound. Shown to induce paraptosis-like cell death. Shown to inhibit P-glycoprotein-mediated transport.
MDL:
MFCD00058400
Molecular Formula:
C25H36O4
Molecular Weight:
400.6
Package Type:
Vial
PG:
III
Precautions:
P301, P310
Product Description:
Cell permeable, irreversible calmodulin antagonist. Acts by covalently binding to a lysine-rich inhibitory site. Inhibits by blocking the activation of the Ca2+/calmodulin-dependent phosphodiesterase [1-3, 5, 6, 9]. Herbicidal mycotoxin [7]. Phytotoxic, antifungal, antibacterial and nematocidal compound [4, 6, 10]. Anticancer compound. Shown to induce paraptosis-like cell death [11, 12]. Shown to inhibit P-glycoprotein-mediated transport [8]. Disruptor of intracellular sulfhydryl proteostasis, leading to ER stress and dilation.
Purity:
>95% (HPLC)
Signal word:
Danger
SMILES:
[H]C(=O)C1=C[C@]2([H])[C@](C)(CC[C@@]22O[C@H](C[C@@H]2C)C=C(C)C)C[C@@]2([H])[C@]1([H])C(=O)C[C@@]2(C)O
Solubility Chemicals:
Soluble in ethanol, methanol, DMSO or dimethylformamide. Poorly soluble in water.
Source / Host:
Isolated from Bipolaris leersia.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Ophiobolin A. A natural product inhibitor of calmodulin: P.C. Leung, et al.; J. Biol. Chem. 259, 2742 (1984) | Role of Calmodulin Inhibition in the Mode of Action of Ophiobolin A: P.C. Leung, et al.; Plant Physiol. 77, 303 (1985) | Characterization of the interaction of ophiobolin A and calmodulin: P.C. Leung, et al.; Int. J. Biochem. 20, 1351 (1988) | Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins: E. Li, et al.; J. Nat. Prod. 58, 74 (1995) | Identification of the binding and inhibition sites in the calmodulin molecule for ophiobolin A by site-directed mutagenesis: T. Kong Au & P. Chow Leung; Plant Physiol. 118, 965 (1998) | The biology of ophiobolins: T.K. Au, et al.; Life Sci. 67, 733 (2000) (Review) | Herbicidal potential of ophiobolins produced by Drechslera gigantea: A. Evidente, et al.; J. Agric. Food Chem. 54, 1779 (2006) | Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products: N. Yoshida, et al.; Food Chem. Toxicol. 44, 2033 (2006) | Calcium depletion and calmodulin inhibition affect the import of nuclear-encoded proteins into plant mitochondria: S. Kuhn, et al.; Plant J. 58, 694 (2009) | Effect of the sesterterpene-type metabolites, ophiobolins A and B, on zygomycetes fungi: K. Krizsan, et al.; FEMS Microbiol. Lett. 313, 135 (2010) | Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity: M. Bury, et al.; Cell Death Dis. 4, e561 (2013) | Ophiobolin A, a sesterterpenoid fungal phytotoxin, displays higher in vitro growth-inhibitory effects in mammalian than in plant cells and displays in vivo antitumor activity: M. Bury, et al.; Int. J. Oncol. 43, 575 (2013) | Ophiobolin A kills human glioblastoma cells by inducing endoplasmic reticulum stress via disruption of thiol proteostasis: I.Y. Kim, et al.; Oncotarget 8, 106740 (2017)
Related Products
| Product Name | Product Code | Supplier | Fuscin | AG-CN2-0138 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoxaline | AG-CN2-0154 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T-2 Toxin | AG-CN2-0473 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||