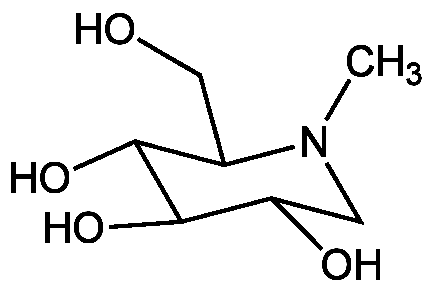
N-Methyl-1-deoxynojirimycin
| Code | Size | Price |
|---|
| BVT-0130-M001 | 1 mg | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
+4°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-Methyl-dNM; N-Methylmoranoline; MOR-14; 1,5-Dideoxy-1,5-(methylimino)-D-glucitol
Appearance:
White solid.
CAS:
69567-10-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light when in solution.
InChi:
InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1
InChiKey:
AAKDPDFZMNYDLR-XZBKPIIZSA-N
Long Description:
Chemical. CAS: 69567-10-8. Formula: C7H15NO4. MW: 177.2. Isolated from Streptomyces sp. alpha-Glucosidase inhibitor. Inhibitor of glycoprotein processing. Strong glycosyltransferase (GTF) inhibitor. Angiogenesis inhibitor. Reduces the size of myocardial infarcts.
MDL:
MFCD00133609
Molecular Formula:
C7H15NO4
Molecular Weight:
177.2
Package Type:
Plastic Vial
Product Description:
alpha-Glucosidase inhibitor. Glucosidase I inhibitor (IC50=0.3µM), 10-fold more potent over 1-Deoxynojirimycin (dNM). Inhibitor of glycoprotein processing. Strong glycosyltransferase (GTF) inhibitor. Angiogenesis inhibitor. Reduces the size of myocardial infarcts.
Purity:
>98% (HPLC, 1H-NMR)
SMILES:
CN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO
Solubility Chemicals:
Soluble in DMSO or water.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation: P.A. Romero, et al.; Virology 130, 238 (1983) | Effect of N-methyl-1-deoxy-nojirimycin on the degradation of cellobiose by Cellulomonas cartalyticum: H. Sahm, et al.; Appl. Microbiol. Biotechnol. 20, 54 (1984) | Purification by affinity chromatography of glucosidase 1, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligasaccharides: H. Hettkamp, et al.; Eur. J. Biochem. 142, 85 (1984) | Comparison between 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin as inhibitors of oligosaccharide processing in intestinal epithelial cells: P.A. Romero, et al.; Biochem. J. 226, 733 (1985) | Different effects of glucosidase inhibitors 1-deoxynojirimycin, N-methyl-1-deoxynojirimycin and castanospermine on the glycosylation of rat alpha1-proteinase inhibitor and alpha1-acid glycoprotein: V. Gross, et al.; Biochem. J. 236, 853 (1986) | Inhibition of myoblast fusion by the glucosidase inhibitor N-methyl-1-deoxynojirimycin, but not by the mannosidase inhibitor 1-deoxymannojirimycin: P.C. Holland, et al.; Biochem. J. 238, 335 (1986) | The glycoprotein-processing inhibitors bromoconduritol and N-methyl-1-deoxynojirimycin alter the adhesion phenotype of skeletal myoblasts: G.C. Trudel, et al.; Biochem. Cell Biol. 68, 1411 (1990) | Glucosidase I, a transmembrane endoplasmic reticular glycoprotein with a luminal catalytic domain: K. Shailubhai, et al.; J. Biol. Chem. 266, 16587 (1991) | Subsite specificity of the active site of glucosyltransferases from Streptococcus sobrinus: K.S. Devulapalle & G. Mooser; J. Biol. Chem. 269, 11967 (1994) | N-methyl-1-deoxynojirimycin (MOR-14), an alpha-glucosidase inhibitor, markedly reduced infarct size in rabbit hearts: M. Arai, et al.; Circ. Res. 97, 1290 (1998) | N-methyl-1-deoxynojirimycin (MOR-14), an alpha-glucosidase inhibitor, markedly improves postischemic left ventricular dysfunction: Y. Nishida, et al.; Heart Vessels 15, 268 (2000) | Combination of N-methyl-1-deoxynojirimycin and ischemic preconditioning markedly reduces the size of myocardial infarcts in rabbits: D.-J. Wu, et al.; Circ. J. 65, 673 (2001) | Role of protein kinase C in the reduction of infarct size by N-methyl-1-deoxynojirimycin, an alpha-1,6-glucosidase inhibitor: M. Arai, et al.; Br. J. Pharmacol. 135, 635 (2001) | Biological study of the angiogenesis inhibitor N-(8-(3-ethynylphenoxy)octyl-1-deoxynojirimycin: Y. Zhao, et al.; Chem. Biol. Drug Des. 75, 570 (2010)